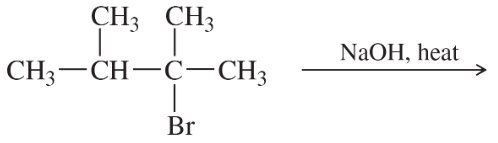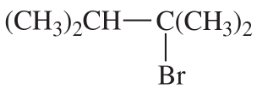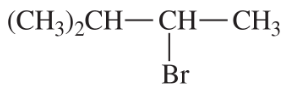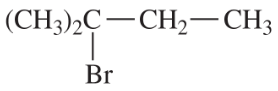Back
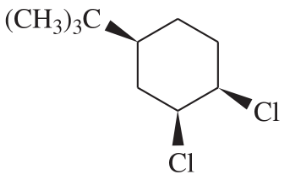
BackProblem 54c
Write a balanced equation for each reaction, showing the major product you expect.
(c)
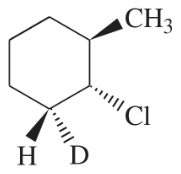
Problem 54d
Write a balanced equation for each reaction, showing the major product you expect.
(d)
Problem 55a,b,c
Predict the dehydrohalogenation product(s) that result when the following alkyl halides are heated in alcoholic KOH. When more than one product is formed, predict the major and minor products.
(a)
(b)
(c)
Problem 55d,e
Predict the dehydrohalogenation product(s) that result when the following alkyl halides are heated in alcoholic KOH. When more than one product is formed, predict the major and minor products.
(d)
(e)
Problem 55f
Predict the dehydrohalogenation product(s) that result when the following alkyl halides are heated in alcoholic KOH. When more than one product is formed, predict the major and minor products.
(f)
Problem 56a,b,c
Using cyclohexane as your starting material, show how you would synthesize each of the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)
a. bromocyclohexane
b. cyclohexene
c. ethoxycyclohexane
Problem 56d,e,f
Using cyclohexane as your starting material, show how you would synthesize each of the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)
d. 3-bromocyclohex-1-ene
e. cyclohexa-1,3-diene
f. cyclohexanol
Problem 57a,b
Show how you would prepare cyclopentene from each compound.
a. cyclopentanol
b. cyclopentyl bromide
Problem 57c
Show how you would prepare cyclopentene from each compound.
c. cyclopentane (not by dehydrogenation)
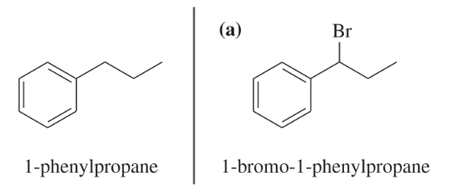
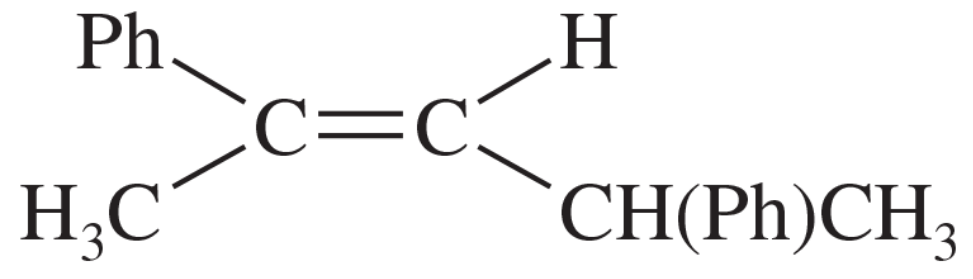
Problem 58a
Show how you would convert (in one or two steps) 1-phenylpropane to the three products shown below. In each case, explain what unwanted reactions might produce undesirable impurities in the product.
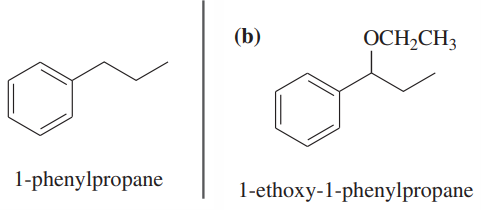
Problem 58b
Show how you would convert (in one or two steps) 1-phenylpropane to the three products shown below. In each case, explain what unwanted reactions might produce undesirable impurities in the product.
Problem 58c
Show how you would convert (in one or two steps) 1-phenylpropane to the three products shown below. In each case, explain what unwanted reactions might produce undesirable impurities in the product.
Problem 59
E1 eliminations of alkyl halides are rarely useful for synthetic purposes because they give mixtures of substitution and elimination products. Explain why the sulfuric acid-catalyzed dehydration of cyclohexanol gives a good yield of cyclohexene even though the reaction goes by an E1 mechanism. (Hint: What are the nucleophiles in the reaction mixture? What products are formed if these nucleophiles attack the carbocation? What further reactions can these substitution products undergo?)
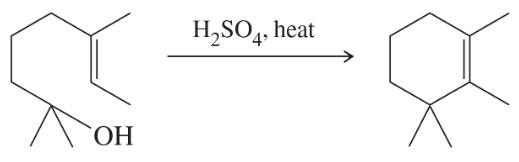
Problem 61a
Propose mechanisms for the following reactions. Additional products may be formed, but your mechanism only needs to explain the products shown.
(a)
Problem 61b
Propose mechanisms for the following reactions. Additional products may be formed, but your mechanism only needs to explain the products shown.
(b)
Problem 61c
Propose mechanisms for the following reactions. Additional products may be formed, but your mechanism only needs to explain the products shown.
(c)
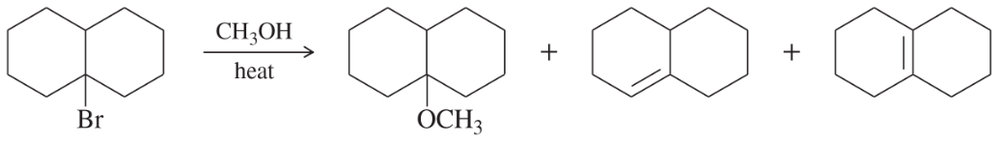
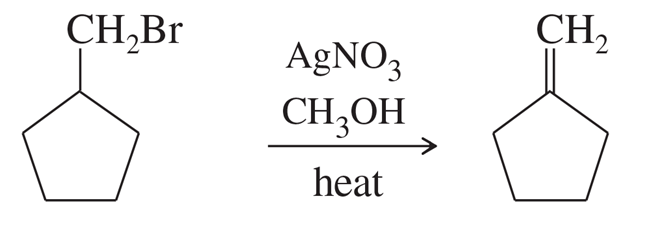
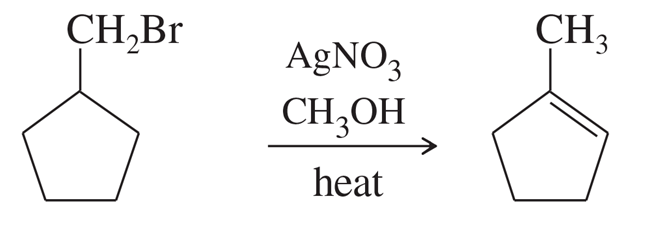
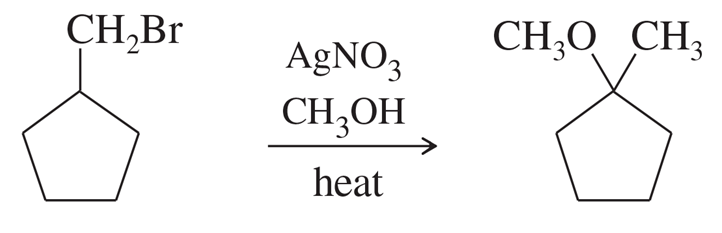
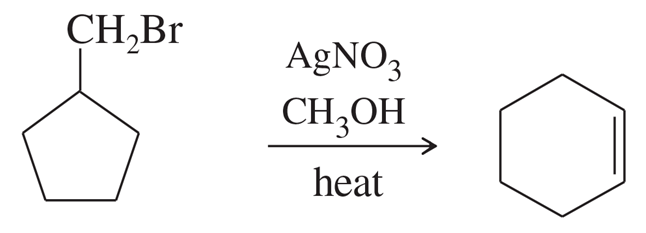
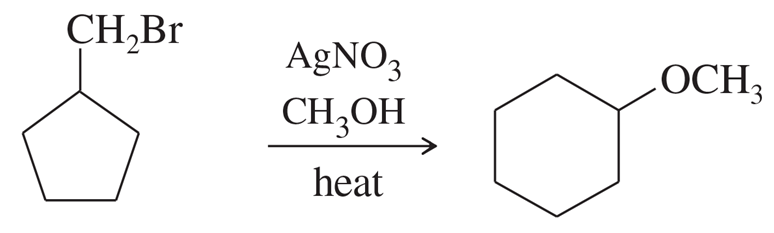
Problem 63a
Silver-assisted solvolysis of bromomethylcyclopentane in methanol gives a complex product mixture of the following five compounds. Propose mechanisms to account for these products.
(a)
Problem 63b
Silver-assisted solvolysis of bromomethylcyclopentane in methanol gives a complex product mixture of the following five compounds. Propose mechanisms to account for these products.
(b)
Problem 63c
Silver-assisted solvolysis of bromomethylcyclopentane in methanol gives a complex product mixture of the following five compounds. Propose mechanisms to account for these products.
(c)
Problem 63d
Silver-assisted solvolysis of bromomethylcyclopentane in methanol gives a complex product mixture of the following five compounds. Propose mechanisms to account for these products.
(d)
Problem 63e
Silver-assisted solvolysis of bromomethylcyclopentane in methanol gives a complex product mixture of the following five compounds. Propose mechanisms to account for these products.
(e)
Problem 64a
Protonation converts the hydroxy group of an alcohol to a good leaving group. Suggest a mechanism for each reaction.
(a)
Problem 64b
Protonation converts the hydroxy group of an alcohol to a good leaving group. Suggest a mechanism for each reaction.
(b)
Problem 65
a. Design an alkyl halide that will give only 2,4-diphenylpent-2-ene upon treatment with potassium tert-butoxide (a bulky base that promotes E2 elimination).
b. What stereochemistry is required in your alkyl halide so that only the following stereoisomer of the product is formed?
Problem 66
A chemist allows some pure (2S,3R)-3-bromo-2,3-diphenylpentane to react with a solution of sodium ethoxide (NaOCH2CH3) in ethanol. The products are two alkenes: A (cis-trans mixture) and B, a single pure isomer. Under the same conditions, the reaction of (2S,3S)-3-bromo-2,3-diphenylpentane gives two alkenes, A (cis-trans mixture) and C. Upon catalytic hydrogenation, all three of these alkenes (A, B, and C) give 2,3-diphenylpentane. Determine the structures of A, B, and C; give equations for their formation; and explain the stereospecificity of these reactions.
Problem 67a
Pure (S)-2-bromo-2-fluorobutane reacts with methoxide ion in methanol to give a mixture of (S)-2-fluoro-2-methoxybutane and three fluoroalkenes.
a. Use mechanisms to show which three fluoroalkenes are formed.
Problem 67b
Pure (S)-2-bromo-2-fluorobutane reacts with methoxide ion in methanol to give a mixture of (S)-2-fluoro-2-methoxybutane and three fluoroalkenes.
b. Propose a mechanism to show how (S)-2-bromo-2-fluorobutane reacts to give (S)-2-fluoro-2-methoxybutane. Has this reaction gone with retention or inversion of configuration?
Problem 68a
When (±)−2,3−dibromobutane reacts with potassium hydroxide, some of the products are (2S,3R)-3-bromobutan-2-ol and its enantiomer and trans-2-bromobut-2-ene. Give mechanisms to account for these products.
Problem 68b
When (±)−2,3−dibromobutane reacts with potassium hydroxide, some of the products are (2S,3R)-3-bromobutan-2-ol and its enantiomer and trans-2-bromobut-2-ene. Why is no cis-2-bromobut-2-ene formed?
Problem 69a
When 2-bromo-3-phenylbutane is treated with sodium methoxide, two alkenes result (by E2 elimination). The Zaitsev product predominates.
a. Draw the reaction, showing the major and minor products.