Back
BackProblem 1a,b,c
Classify each compound as an alkyl halide, a vinyl halide, or an aryl halide.
(a) CH3CHCFCH3
(b) (CH3)3CBr
(c) CH3CCl3
Problem 1d,e,f
Classify each compound as an alkyl halide, a vinyl halide, or an aryl halide.
Problem 2a,b
Give the structures of the following compounds.
a. methylene iodide
b. carbon tetrabromide
Problem 2e,f
Give the structures of the following compounds.
e. 2-bromo-3-ethyl-2-methylhexane
f. isobutyl bromide
Problem 2g,h
Give the structures of the following compounds.
g. cis-1-fluoro-3-(fluoromethyl)cyclohexane
h. tert-butyl chloride
Problem 3a
For each of the following compounds,
1. give the IUPAC name.
2. give the common name (if possible).
3. classify the compound as a methyl, primary, secondary, or tertiary halide.
(a) (CH3)2CHCH2Cl
Problem 3b
For each of the following compounds,
1. give the IUPAC name.
2. give the common name (if possible).
3. classify the compound as a methyl, primary, secondary, or tertiary halide.
b. (CH3)3CBr
Problem 3c,d
For each of the following compounds,
1. give the IUPAC name.
2. give the common name (if possible).
3. classify the compound as a methyl, primary, secondary, or tertiary halide.
c.
d.
Problem 3e
For each of the following compounds,
1. give the IUPAC name.
2. give the common name (if possible).
3. classify the compound as a methyl, primary, secondary, or tertiary halide.
e.
Problem 3f
For each of the following compounds,
1. give the IUPAC name.
2. give the common name (if possible).
3. classify the compound as a methyl, primary, secondary, or tertiary halide.
(f)
Problem 4a
Kepone, aldrin, and chlordane are synthesized from hexachlorocyclopentadiene and other five-membered-ring compounds. Show how these three pesticides are composed of two five-membered rings.
Problem 5a,b
For each pair of compounds, predict which one has the higher molecular dipole moment, and explain your reasoning.
a. ethyl chloride or ethyl iodide
b. 1-bromopropane or cyclopropane
Problem 5c,d
For each pair of compounds, predict which one has the higher molecular dipole moment, and explain your reasoning.
c. cis-2,3-dibromobut-2-ene or trans-2,3-dibromobut-2-ene
d. cis-1,2-dichlorocyclobutane or trans-1,3-dichlorocyclobutane
- The reaction of an amine with an alkyl halide gives an ammonium salt. R3N amine + R'—X alkyl halide —> R3(N+)—R' X- ammonium salt The rate of this SN2 reaction is sensitive to the polarity of the solvent. 1. Draw an energy diagram for this reaction in a nonpolar solvent and another in a polar solvent. 2. Consider the nature of the transition state, and explain why this reaction should be sensitive to the polarity of the solvent. 3. Predict whether it will be faster or slower in a more polar solvent.
Problem 6

- The reaction of an amine with an alkyl halide gives an ammonium salt. R3N amine + R'—X alkyl halide —> R3(N+)—R' X- ammonium salt The rate of this SN2 reaction is sensitive to the polarity of the solvent. 1. Draw an energy diagram for this reaction in a nonpolar solvent and another in a polar solvent. 2. Consider the nature of the transition state, and explain why this reaction should be sensitive to the polarity of the solvent. 3. Predict whether it will be faster or slower in a more polar solvent.
Problem 6
Problem 6a,b
a. Propose a mechanism for the following reaction:
b. Use the bond-dissociation enthalpies given in Table 4-2 (page 167) to calculate the value of ΔH° for each step shown in your mechanism. (The BDE for CH2=CHCH2―Br is about 280 kJ/mol, or 67 kcal/mol.) Calculate the overall value of ΔH° for the reaction. Are these values consistent with a rapid free-radical chain reaction?
Problem 6a,b,c
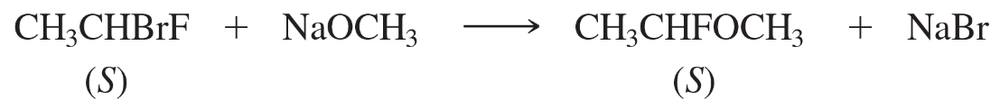
Under appropriate conditions, (S)-1-bromo-1-fluoroethane reacts with sodium methoxide to give pure (S)-1-fluoro-1-methoxyethane.
a. Why is bromide rather than fluoride replaced?
b. Draw perspective structures (as shown on the previous page for 2-bromobutane) for the starting material, the transition state, and the product.
c. Does the product show retention or inversion of configuration? d. Is this result consistent with reaction by the SN2 mechanism?
Problem 6a
For each pair of compounds, predict which compound has the higher boiling point. Check [TABLE 6-2] to see if your prediction was right; then explain why that compound has the higher boiling point.
a. isopropyl bromide and n-butyl bromide
Problem 6b
For each pair of compounds, predict which compound has the higher boiling point. Check [TABLE 6-2] to see if your prediction was right; then explain why that compound has the higher boiling point.
b. isopropyl chloride and tert-butyl bromide
Problem 6c
For each pair of compounds, predict which compound has the higher boiling point. Check [TABLE 6-2] to see if your prediction was right; then explain why that compound has the higher boiling point.
c. 1-bromobutane and 1-chlorobutane
Problem 6-15a
Show how you might use SN2 reactions to convert 1-chlorobutane into the following compounds.
a. butan-1-ol
Problem 6-15b
Show how you might use SN2 reactions to convert 1-chlorobutane into the following compounds.
b. 1-fluorobutane
Problem 9a
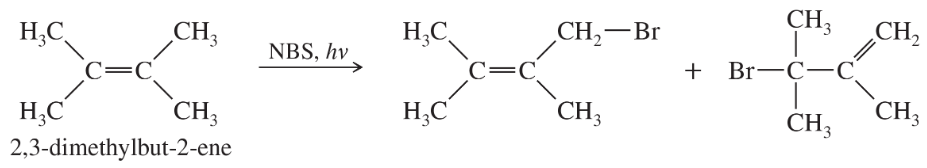
The light-initiated reaction of 2,3-dimethylbut-2-ene with N-bromosuccinimide (NBS) gives two products:
a. Give a mechanism for this reaction, showing how the two products arise as a consequence of the resonance-stabilized intermediate.
Problem 9b
The light-initiated reaction of 2,3-dimethylbut-2-ene with N-bromosuccinimide (NBS) gives two products:
b. The bromination of cyclohexene using NBS gives only one major product, as shown on the previous page. Explain why there is no second product from an allylic shift.
Problem 10a,b
Show how free-radical halogenation might be used to synthesize the following compounds. In each case, explain why we expect to get a single major product.
(a) 1-chloro-2,2-dimethylpropane (neopentyl chloride)
(b) 2-bromo-2-methylbutane
Problem 10c
Show how free-radical halogenation might be used to synthesize the following compounds. In each case, explain why we expect to get a single major product.
(c)
Problem 10d
Show how free-radical halogenation might be used to synthesize the following compounds. In each case, explain why we expect to get a single major product.
(d)
Problem 11a
Classify each reaction as a substitution, an elimination, or neither. Identify the leaving group in each reaction, and the nucleophile in substitutions.
a.
Problem 11b
Classify each reaction as a substitution, an elimination, or neither. Identify the leaving group in each reaction, and the nucleophile in substitutions.
b.
Problem 11c
Classify each reaction as a substitution, an elimination, or neither. Identify the leaving group in each reaction, and the nucleophile in substitutions.
c.