Ch. 23 - Benzene I: Aromatic Stability and Substitution Reactions
 Back
BackProblem 2
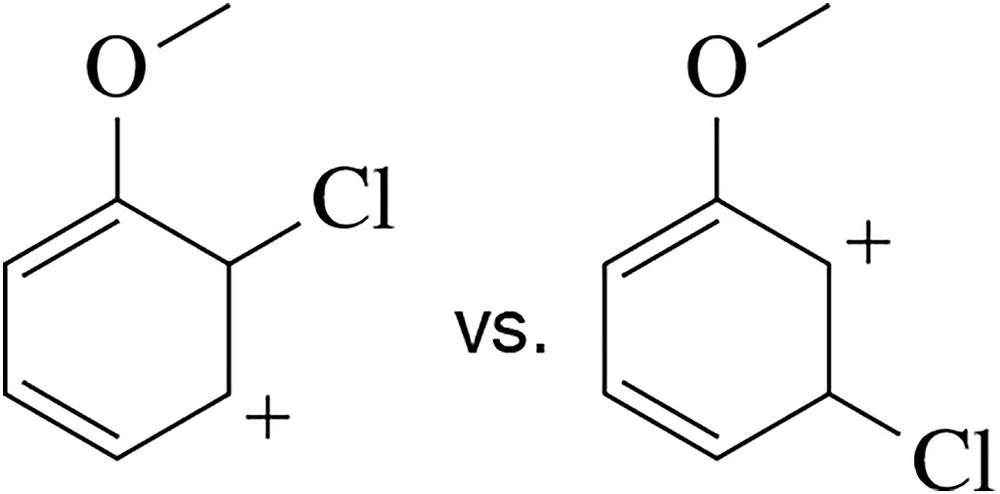
Draw resonance structures to identify which of the following is the more stable carbocation.
Problem 5a
Suggest an arrow-pushing mechanism that accounts for the formation of the following Lewis acid–Lewis base complexes. Label the Lewis acid and Lewis base in each.
(a)
Problem 5b
Suggest an arrow-pushing mechanism that accounts for the formation of the following Lewis acid–Lewis base complexes. Label the Lewis acid and Lewis base in each.
(b)
Problem 7
The average C―C single bond length is 1.53 Å, and the average C=C double bond length is 1.31 Å. All of the C―C bonds in benzene are the same length (1.42 Å). Explain.