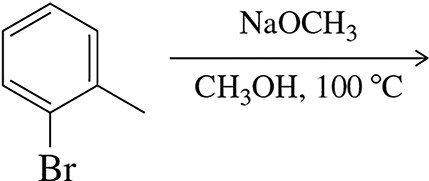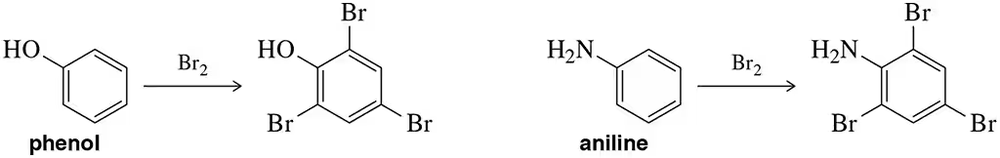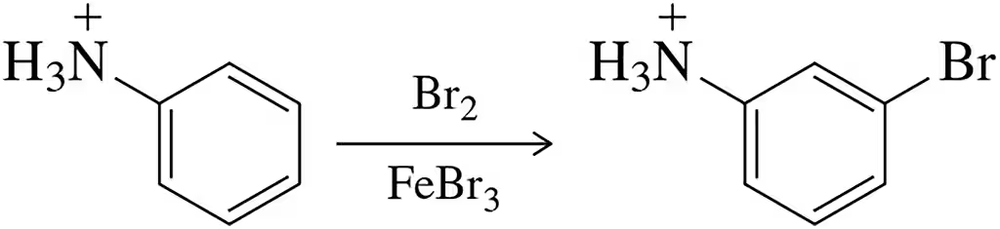Back
BackProblem 56b
Predict the product(s) of the following nucleophilic aromatic substitution reactions occurring by the benzyne mechanism.
(b)
Problem 57a
Predict the products of the following reactions.
(a)
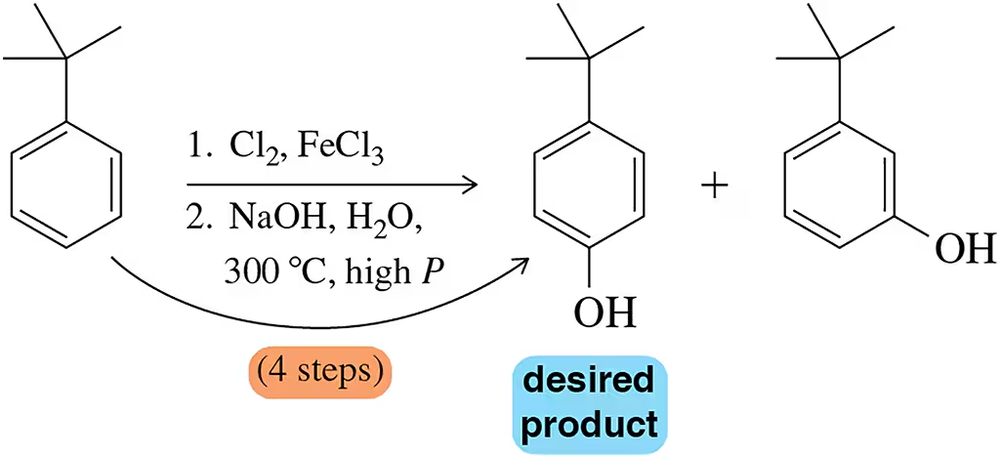
Problem 58
Conversion of t-butylbenzene to a phenol results in two products when the reaction occurs through the benzyne mechanism. Devise a sequence involving a diazonium ion in which only one phenol product is produced.
Problem 59a
Predict the products of the following reactions.
(a)
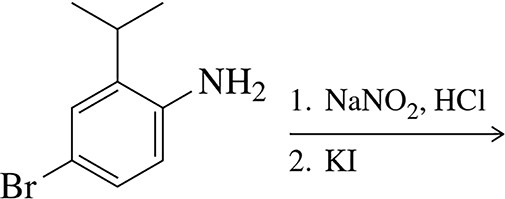
Problem 59b
Predict the products of the following reactions.
(b)
Problem 60a
Suggest two coupling partners that could be used to synthesize the compounds shown making the indicated C―C bonds.
(a)
Problem 61a
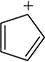
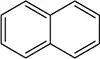
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For aromatic molecules, solve for n in Hückel’s rule. For all other molecules, explain which rule of aromaticity is being broken.
(a)
Problem 61b
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For aromatic molecules, solve for n in Hückel’s rule. For all other molecules, explain which rule of aromaticity is being broken.
(b)
Problem 61c
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For aromatic molecules, solve for n in Hückel’s rule. For all other molecules, explain which rule of aromaticity is being broken.
(c)
Problem 61d
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For aromatic molecules, solve for n in Hückel’s rule. For all other molecules, explain which rule of aromaticity is being broken.
(d)
Problem 61e
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For aromatic molecules, solve for n in Hückel’s rule. For all other molecules, explain which rule of aromaticity is being broken.
(e)
Problem 61f
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For aromatic molecules, solve for n in Hückel’s rule. For all other molecules, explain which rule of aromaticity is being broken.
(f)
Problem 61g
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For aromatic molecules, solve for n in Hückel’s rule. For all other molecules, explain which rule of aromaticity is being broken.
(g)
Problem 61h
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For aromatic molecules, solve for n in Hückel’s rule. For all other molecules, explain which rule of aromaticity is being broken.
(h)
Problem 61i
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For aromatic molecules, solve for n in Hückel’s rule. For all other molecules, explain which rule of aromaticity is being broken.
(i)
Problem 62a
Use a Frost circle diagram to construct the molecular orbital diagram for the molecules shown. Would you expect them to be aromatic or antiaromatic?
(a)
Problem 62b
Use a Frost circle diagram to construct the molecular orbital diagram for the molecules shown. Would you expect them to be aromatic or antiaromatic?
(b)
Problem 62c
Use a Frost circle diagram to construct the molecular orbital diagram for the molecules shown. Would you expect them to be aromatic or antiaromatic?
(c)
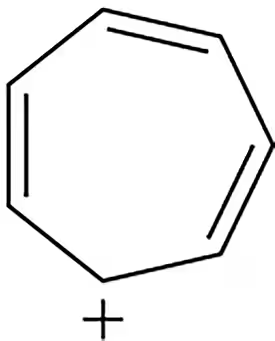
Problem 63
Using a Frost circle, a student drew the molecular orbital picture shown for the cyclopentadienyl anion, determining it to be antiaromatic. Do you agree with this conclusion? Why or why not?
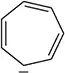
Problem 64
Despite having only sp2-hybridized carbons and having 10 electrons (4n + 2) , the annulene shown is not aromatic. Why?
Problem 66
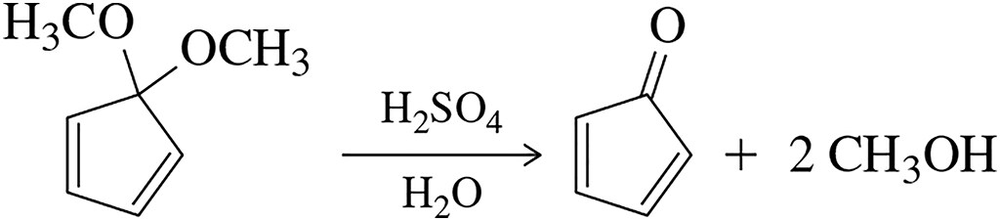
In Section 17.7.4, we studied the acid-catalyzed hydrolysis of acetals. The acetal shown here resists hydrolysis by the mechanism in Figure 17.63. Why? [Draw the intermediates as if the reaction would occur, then analyze the intermediates for any problems.]
Problem 68
Molecule A is significantly more water soluble than molecule B. Justify this observation.
Problem 72a
Predict the major product(s) that would result when molecules (a)–(i) are allowed to react under the following conditions. (iv) chlorocyclopentane, AlCl3, (v) 1-chloro-1-methylcyclohexane , AlCl3, (vi) PhCOCl, AlCl3. If no reaction will occur, indicate by writing NR.
(a)
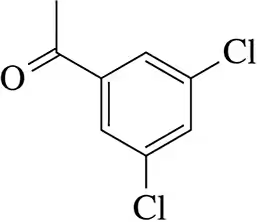
Problem 74c
Suggest a synthesis of each of the molecules shown beginning with benzene.
(c)
Problem 75
Bromination of phenol or aniline does not require the use of a Lewis acid catalyst and often results in trihalogenation. Why?
Problem 77
Protonation of aniline slows electrophilic aromatic substitution and directs electrophiles to the meta position. Why?
Problem 80
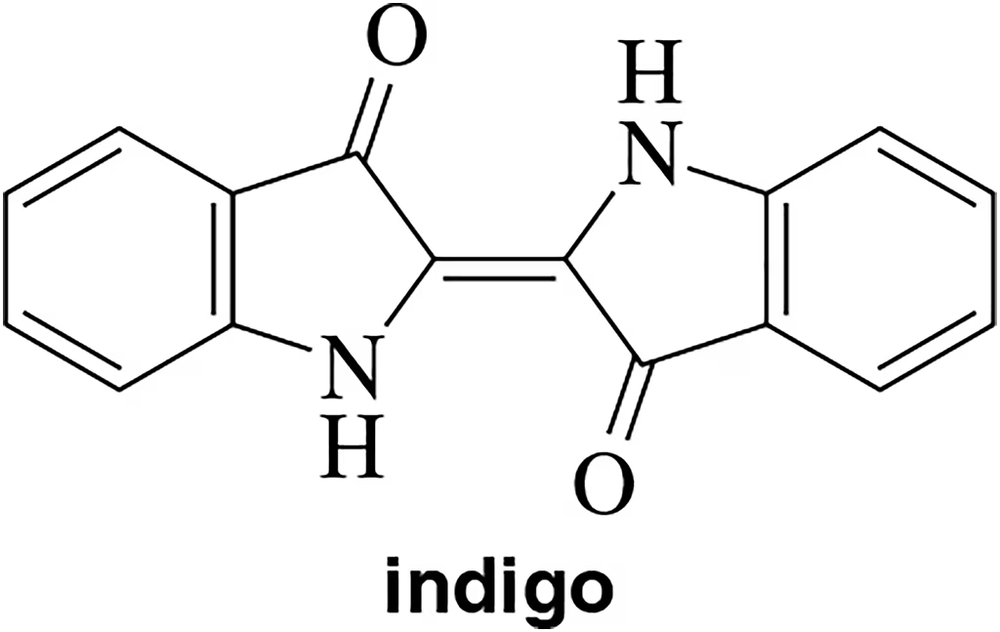
Indigo is a dye that was originally isolated from coal tar. In 1905, Johann Friedrich Wilhelm Adolf von Baeyer won a Nobel Prize for a method that allowed indigo to be isolated from plants [a green chemist ahead of his time.] If you nitrated indigo using the reaction learned in this chapter, at which carbon would you expect the nitro group to attach?
Problem 81
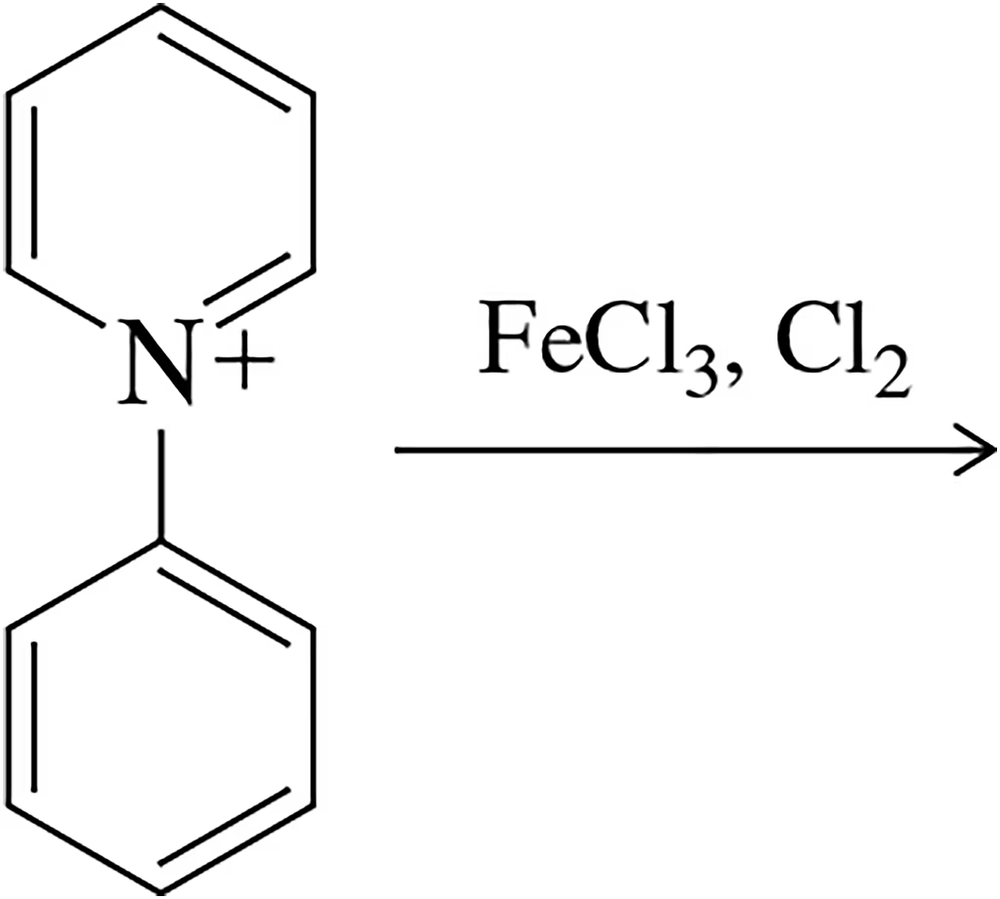
Would you expect chlorination to occur ortho, para, or meta to the pyridinium ion in the following molecule? Explain your answer.
Problem 83
Fluoxetine, an antidepressant, is better known as Prozac®. Suggest reagents that could be used to make the indicated C–O bond of fluoxetine. [Note that CF3, an electron-withdrawing group, is para to where the new bond will be formed.]
Problem 84
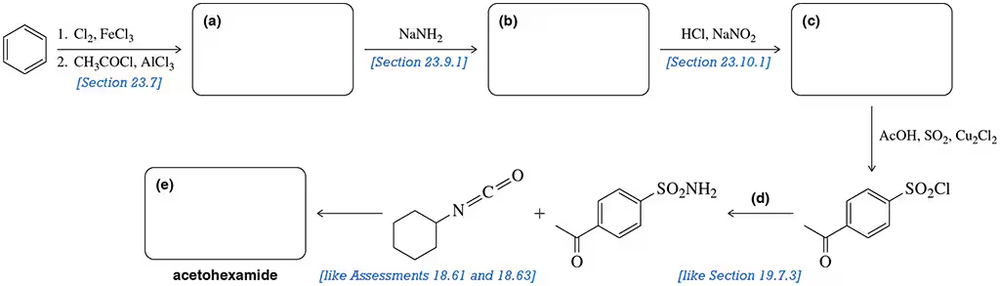
Acetohexamide is used to treat type 2 diabetes that cannot be well controlled by diet alone. Fill in the blanks of the synthetic scheme used to synthesize acetohexamide. [Hints have been provided below each step.]