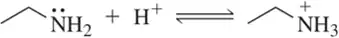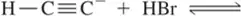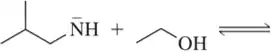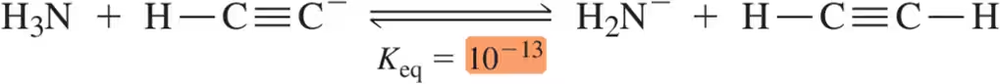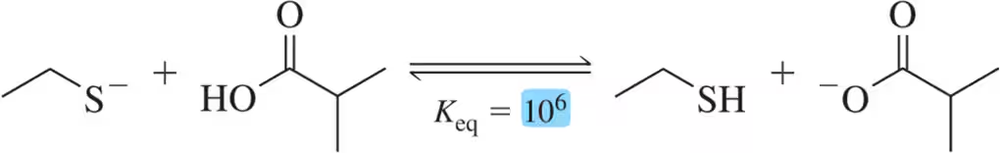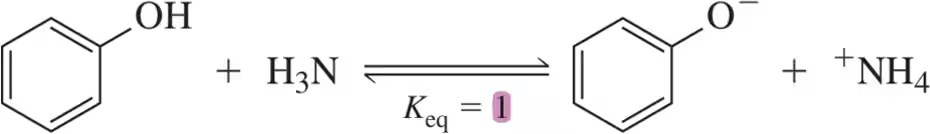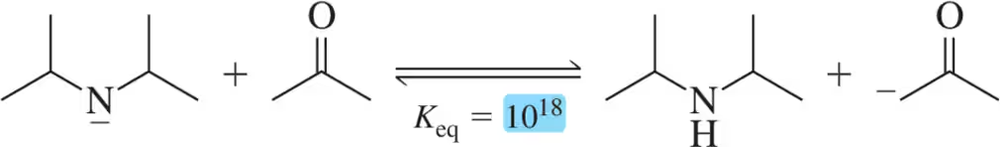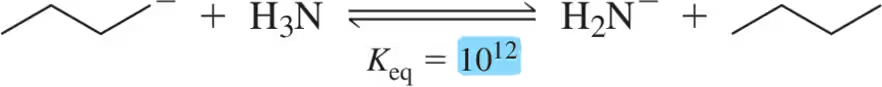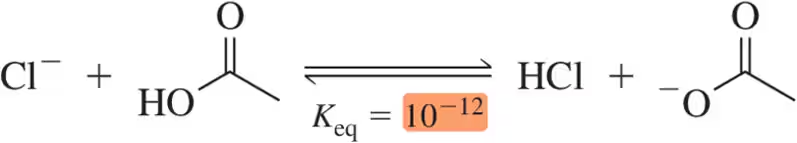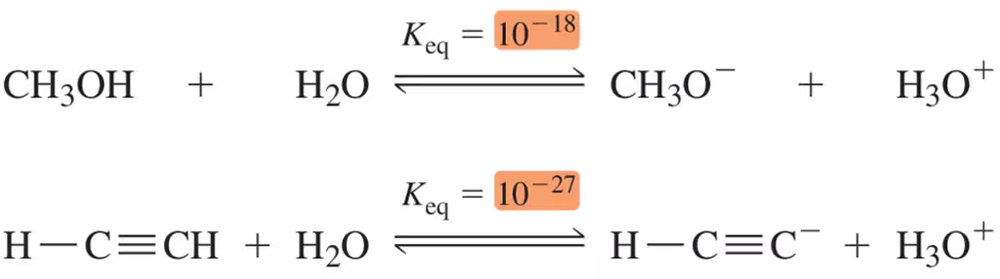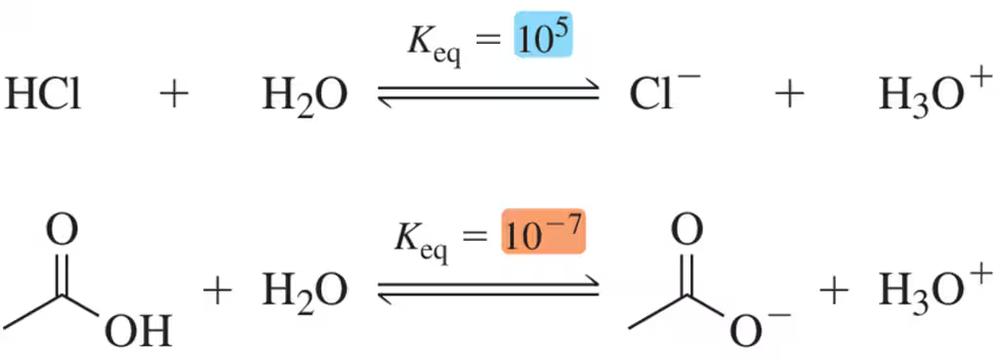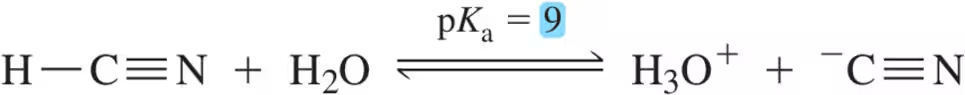Back
BackProblem 17b
Provide an arrow-pushing mechanism for the following hypothetical base half-reactions.
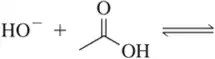
(b)
Problem 17c
Provide an arrow-pushing mechanism for the following hypothetical base half-reactions.
(c)
Problem 18c
Provide an arrow-pushing mechanism for the following acid–base reactions.
(c)
Problem 18d
Provide an arrow-pushing mechanism for the following acid–base reactions.
(d)
Problem 19
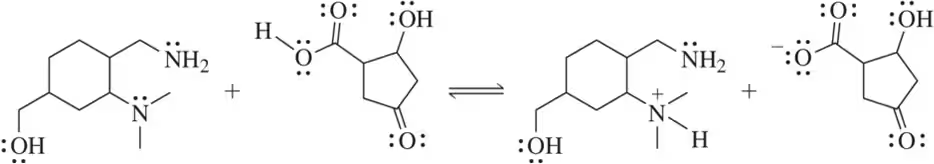
Acid–base reactions are reversible. Show a mechanism for the reverse of the reactions in Assessment 4.18.
Problem 20a(iv,v)
In the following reactions,
(iv) provide an arrow-pushing mechanism of the proton transfer that will occur, and
(v) predict the product of the reactions. [You'll need to provide the lone pairs here.]
(a)
Problem 20a(i,ii,iii)
In the following reactions,
(i) identify the acid and base,
(ii) identify the most electron-rich atom in the base,
(iii) identify the most acidic hydrogen in the acid,
(a)
Problem 20b(i,ii,iii)
In the following reactions,
(i) identify the acid and base,
(ii) identify the most electron-rich atom in the base,
(iii) identify the most acidic hydrogen in the acid,
(b)
Problem 20b(iv,v)
In the following reactions,
(iv) provide an arrow-pushing mechanism of the proton transfer that will occur, and
(v) predict the product of the reactions. [You'll need to provide the lone pairs here.]
(b)
Problem 20c(i,ii,iii)
In the following reactions,
(i) identify the acid and base,
(ii) identify the most electron-rich atom in the base,
(iii) identify the most acidic hydrogen in the acid,
(c)
Problem 20c(iv,v)
In the following reactions,
(iv) provide an arrow-pushing mechanism of the proton transfer that will occur, and
(v) predict the product of the reactions. [You'll need to provide the lone pairs here.]
(c)
Problem 20d
In the following reactions,
(i) identify the acid and base,
(ii) identify the most electron-rich atom in the base,
(iii) identify the most acidic hydrogen in the acid,
(iv) provide an arrow-pushing mechanism of the proton transfer that will occur, and
(v) predict the product of the reactions. [You'll need to provide the lone pairs here.]
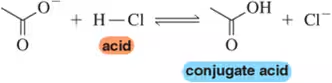
(d) H2O + HCl ⇌
Problem 21c
Write the Keq expression for the following acid–base reactions. [You don't need to calculate Keq here.]
(c)
Problem 22a
Given the value of Keq for the following acid–base reactions, identify the weakest acid and the weakest base.
(a)
Problem 22b
Given the value of Keq for the following acid–base reactions, identify the weakest acid and the weakest base.
(b)
Problem 22c
Given the value of Keq for the following acid–base reactions, identify the weakest acid and the weakest base.
(c)
Problem 23a
Given the Keq values for the following acid–base reactions, identify the strongest acid and the strongest base.
(a)
Problem 23b
Given the Keq values for the following acid–base reactions, identify the strongest acid and the strongest base.
(b)
Problem 23c
Given the Keq values for the following acid–base reactions, identify the strongest acid and the strongest base.
(c)
Problem 24
An unknown base (B⁻) has been identified as very weak. What does this tell you about the strength of its conjugate acid, HB? Is it stable or unstable? Is it reactive or unreactive?
Problem 25a
An unknown acid (HA) has been identified as very strong. What does this tell you about the stability of the conjugate base, A⁻? Is it strong or weak? Is it reactive or unreactive?
Problem 26
Design an acid–base extraction scheme to separate a mixture of the basic amine N,N-dimethylaniline and naphthalene.
Problem 28
Given the following Keq values, how much stronger of an acid is methanol (CH3OH) than acetylene (HC≡CH)?
Problem 29
Given the following Keq values, how much stronger of a base is acetate (CH3CO2-) than chloride (Cl⁻)?
Problem 30a
Given that the indicated pKa values correspond to the acid dissociation reactions shown, calculate the ratio of acid to conjugate base for the reactions shown.
(a)
Problem 30b
Given that the indicated pKa values correspond to the acid dissociation reactions shown, calculate the ratio of acid to conjugate base for the reactions shown.
(b)
Problem 31
Hydrogen gas (H2) has a relatively high pKa value. Is it a stable or unstable acid? Do you expect it to participate in acid–base reactions?
Problem 32
How do you know that a proton with a low pKa value is acidic (besides 'I just remember')?
Problem 33
If a base has a conjugate acid with a high pKa value, is it stable or unstable? How do you know this is true (besides 'I just remember')?
Problem 36a
Using qualitative reasoning for the acid–base reactions shown,
(i) which is stronger, the acid or the conjugate acid?
(ii) Which side of the reaction is favored?
(iii) Would you expect a Keq greater than, equal to, or less than 1?
(a)