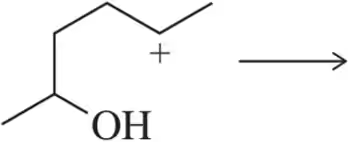Back
BackProblem 63d
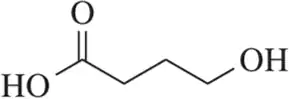
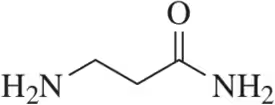
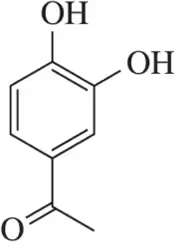
Identify the stronger base in each pair. Explain your choice. Citing pKa values is not an acceptable answer.
(d)
Problem 64a
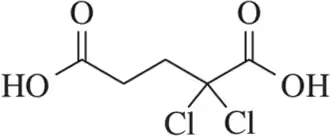
Identify the stronger acid in each pair. Explain your choice. Citing pKa values is not an acceptable answer.
(a)
Problem 65
Benzoic acid and phenol are insoluble in water. When sodium bicarbonate is added to the water, benzoic acid dissolves, but phenol does not. The addition of sodium hydroxide causes both to dissolve. On the basis of these observations, estimate the pKa of phenol. [The pKa of H2CO3 is 6.4.]
Problem 66a
For each of the following molecules, predict the product that would form upon reaction of a single equivalent of a strong base.
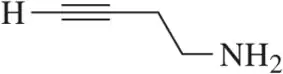
(a)
Problem 66b
For each of the following molecules, predict the product that would form upon reaction of a single equivalent of a strong base.
(b)
Problem 66c
For each of the following molecules, predict the product that would form upon reaction of a single equivalent of a strong base.
(c)
Problem 66d
For each of the following molecules, predict the product that would form upon reaction of a single equivalent of a strong base.
(d)
Problem 66e
For each of the following molecules, predict the product that would form upon reaction of a single equivalent of a strong base.
(e)
Problem 66f
For each of the following molecules, predict the product that would form upon reaction of a single equivalent of a strong base.
(f)
Problem 67
Suggest a scheme by which N-methylmorpholine, salicylic acid, and naphthalene can be separated.
Problem 68
A medicinal chemist needed to deprotonate acetylene ( HC≡CH ) for use in a coupling reaction. Among the options given, which base(s) could be used for this process?
Problem 69
Amino acids exist predominantly in one of the following forms. Which is it? Explain your answer.
Problem 70a
Using pKa values for the conjugate acids of the bases on each side of the reaction arrow, identify which side of the equilibrium would be favored in the following hypothetical reactions.
(a)
Problem 70c
Using pKa values for the conjugate acids of the bases on each side of the reaction arrow, identify which side of the equilibrium would be favored in the following hypothetical reactions.
(c)
Problem 72c
Predict the product of the following Lewis acid–Lewis base reactions.
(c)
Problem 72d
Predict the product of the following Lewis acid–Lewis base reactions.
(d) [an intramolecular reaction]
Problem 73c
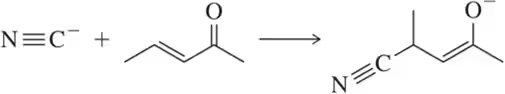
Suggest an arrow-pushing mechanism for each of the following acid–base reactions
(c)
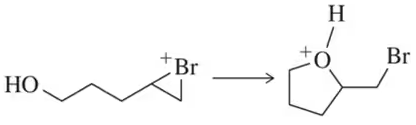
Problem 73d
Suggest an arrow-pushing mechanism for each of the following acid–base reactions.
(d) [an intramolecular reaction]
Problem 74
One of the more reactive species we will study in organic chemistry is the carbene (molecular formula of CH2). The carbene can react as both a Lewis acid and a Lewis base. Explain these properties.
Problem 75
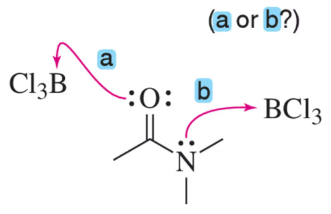
BCl3 has an empty p orbital, so it is a strong Lewis acid. Would you expect an amide to react with BCl3 at nitrogen or oxygen?
Problem 77
Consider the following drugs used to treat the indicated diseases. Would you expect the activity of these drugs to be impacted by a patient taking other medicines for acid reflux disease?
Problem 78
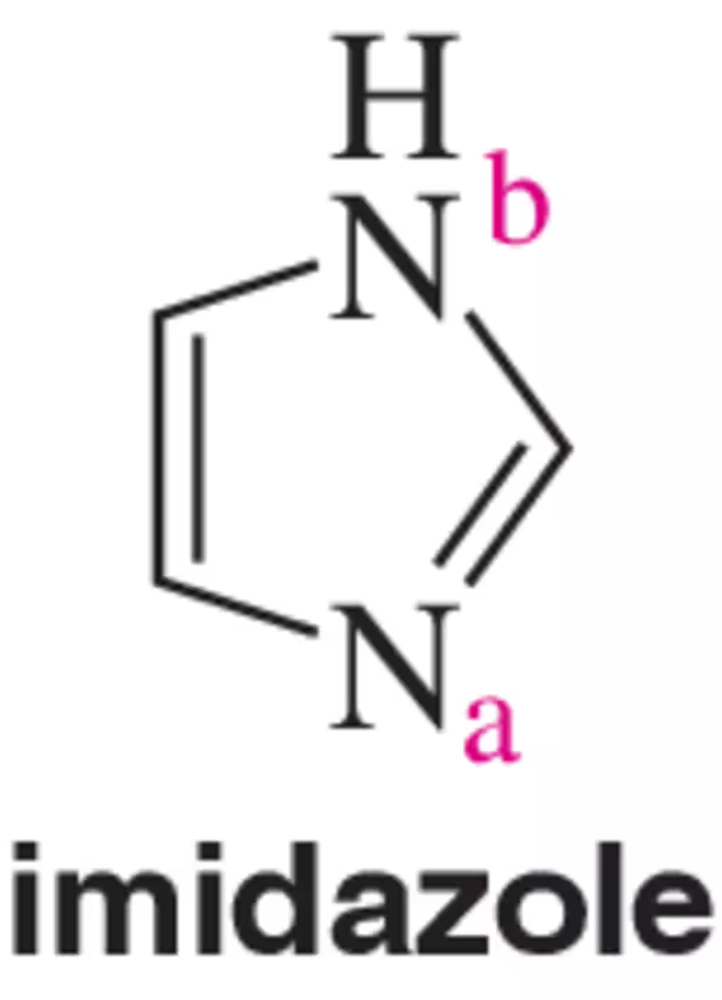
Imidazole has two potentially basic sites (a and b). Of the two, where would you expect a proton to add preferentially?
Problem 79c
In addition to radicals, anions, and cations, a fourth class of reactive intermediates is carbenes. A neutral species, the simplest carbene has a molecular formula of CH2.
(c) Carbenes are Lewis acids. Based on your answers to (a) and (b), explain this observation.
Problem 79d
In addition to radicals, anions, and cations, a fourth class of reactive intermediates is carbenes. A neutral species, the simplest carbene has a molecular formula of CH2.
(d) Carbenes are also Lewis bases. Based on your answers to (a) and (b), explain this observation.