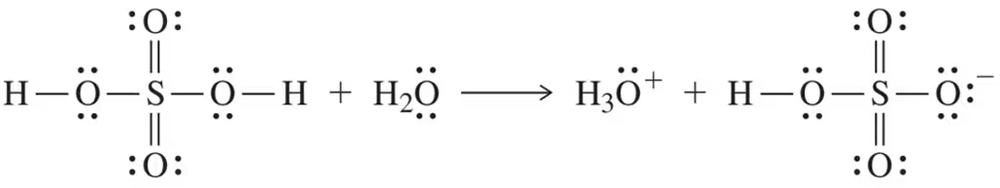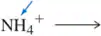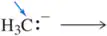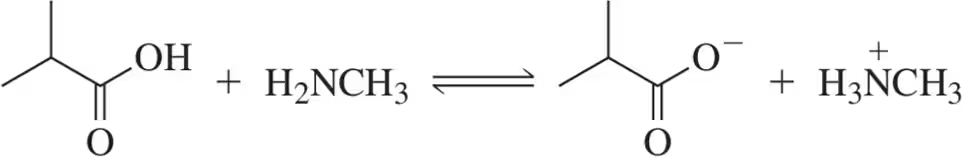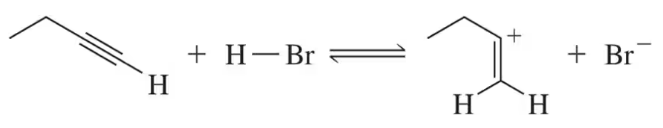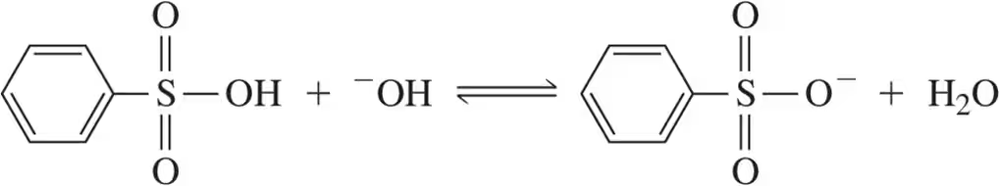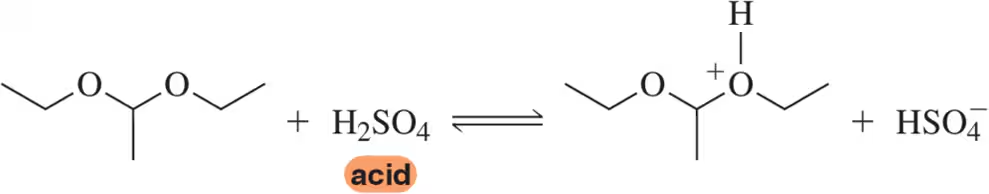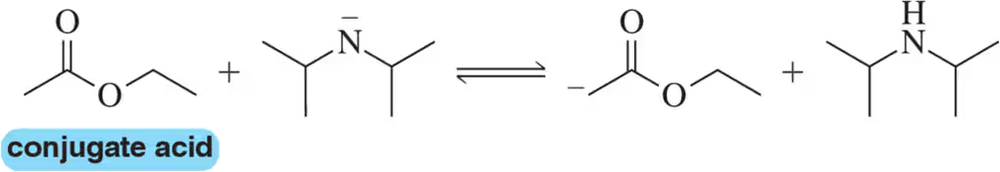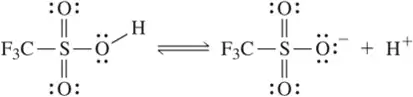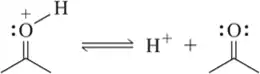Back
BackProblem 2
Which is more stable, free hydrogen atoms or the diatomic H2 molecule? Why do you know this to be true?
Problem 3
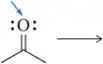
Draw the Lewis structure of the methoxide ion ( CH3O-). Draw the Lewis structure of a proton (H+) . To which atom of methoxide would you expect a proton to add?
Problem 4.24
An unknown base (B⁻) has been identified as very weak. What does this tell you about the strength of its conjugate acid, HB? Is it stable or unstable? Is it reactive or unreactive?
Problem 4.24
An unknown base (B⁻) has been identified as very weak. What does this tell you about the strength of its conjugate acid, HB? Is it stable or unstable? Is it reactive or unreactive?
Problem 4.62e5
For the following acid–base pairs, (v) show a mechanism for the reaction;
(e)
Problem 5b
Draw the molecular orbital picture of the following molecules and ions. In each, how many electrons are in the p orbital on the central atom? (b) BH3
Problem 5c
Draw the molecular orbital picture of the following molecules and ions. In each, how many electrons are in the p orbital on the central atom? (c) AlCl3
Problem 6b
In each pair of atoms, which has the larger atomic radius? Which is more electronegative?
(b) C vs. O
Problem 6c
In each pair of atoms, which has the larger atomic radius? Which is more electronegative?
(c) O vs. S
Problem 7a
Sodium amide (NaNH2) dissociates to give a sodium cation (Na+) and amide ion (NH2-) a very strong base. In the following three equations, identify which definition of base is being exemplified.
(a)
Problem 7b
Sodium amide (NaNH₂) dissociates to give a sodium cation (Na+) and amide ion (NH2–) a very strong base. In the following three equations, identify which definition of base is being exemplified.
(b)
Problem 7c
Sodium amide (NaNH2) dissociates to give a sodium cation (Na+) and amide ion (NH2-) a very strong base. In the following three equations, identify which definition of base is being exemplified.
(c)
Problem 8a
Sulfuric acid is a very strong acid. Show how the following equation can be used to explain that sulfuric acid is at once an Arrhenius, Brønsted–Lowry, and Lewis acid.
Problem 9a
What is the conjugate base of each of the following acids? [The most acidic proton is indicated.]
(a)
Problem 9c
What is the conjugate base of each of the following acids? [The most acidic proton is indicated.]
(c)
Problem 9d
What is the conjugate base of each of the following acids? [The most acidic proton is indicated.]
(d)
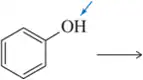
Problem 10a
What is the conjugate acid of each of the following bases? [The most basic atom is indicated.]
(a)
Problem 10d
What is the conjugate acid of each of the following bases? [The most basic atom is indicated.]
(d)
Problem 11c
Identify the acid from which the following conjugate bases were formed. [The most basic atom is indicated.]
(c)
Problem 11d
Identify the acid from which the following conjugate bases were formed. [The most basic atom is indicated.]
(d)
Problem 12c
Identify the base from which the following conjugate acids were formed. [The most acidic proton is indicated.]
(c)
Problem 13
When an atom loses a proton in an acid–base reaction, its formal charge is decreased by one. Thinking about the equation for calculating formal charge, explain this observation in your own words.
Problem 14a
Using the convention that the acid and base are on the left side of the chemical equation, label the acid, base, conjugate acid, and conjugate base in the following reactions.
(a)
Problem 14b
Using the convention that the acid and base are on the left side of the chemical equation, label the acid, base, conjugate acid, and conjugate base in the following reactions.
(b)
Problem 14c
Using the convention that the acid and base are on the left side of the chemical equation, label the acid, base, conjugate acid, and conjugate base in the following reactions.
(c)
Problem 15a
Based on the one species that is identified for you, label the remaining molecules as acid, base, conjugate acid, or conjugate base.
(a)
Problem 15b
Based on the one species that is identified for you, label the remaining molecules as acid, base, conjugate acid, or conjugate base.
(b)
Problem 15c
Based on the one species that is identified for you, label the remaining molecules as acid, base, conjugate acid, or conjugate base.
(c)
Problem 16a
Provide an arrow-pushing mechanism for the following hypothetical acid half-reactions. [These are only intended to help you learn about arrow pushing in acid–base reactions.]
(a)
Problem 16b
Provide an arrow-pushing mechanism for the following hypothetical acid half-reactions. [These are only intended to help you learn about arrow pushing in acid–base reactions.]
(b)