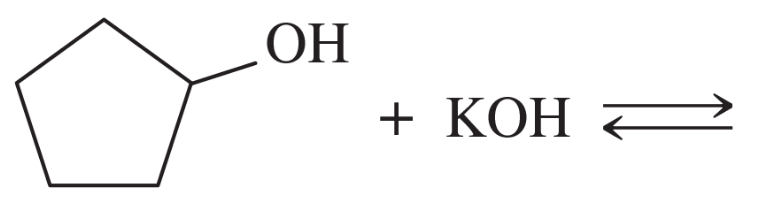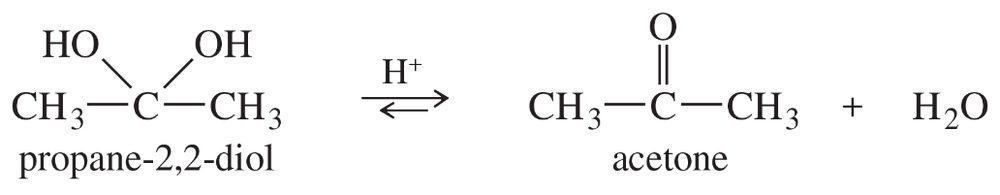Back
BackProblem 40c
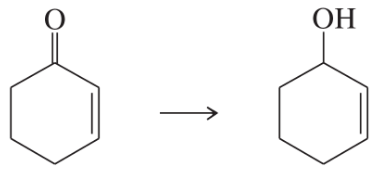
Show how you would accomplish the following transformations. You may use any additional reagents you need.
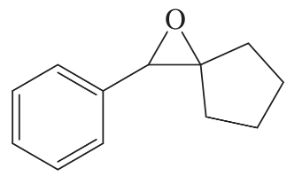
(c)
Problem 40d
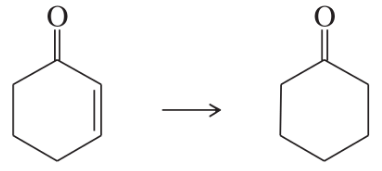
Show how you would accomplish the following transformations. You may use any additional reagents you need.
(d)
Problem 41a
Show how you would synthesize the following:
a. 2-phenylethanol by the addition of formaldehyde to a suitable Grignard reagent
Problem 41c
Show how you would synthesize the following:
c. cyclohexylmethanol from an alkyl halide using an SN2 reaction
Problem 41e
Show how you would synthesize the following:
e. cis-pent-2-en-1-thiol from a suitable alkenyl halide
Problem 41f
Show how you would synthesize the following:
f. 2,5-dimethylhexane from a four-carbon alkyl halide
Problem 42d
Complete the following acid–base reactions. In each case, indicate whether the equilibrium favors the reactants or the products, and explain your reasoning.
(d)
Problem 42e
Complete the following acid–base reactions. In each case, indicate whether the equilibrium favors the reactants or the products, and explain your reasoning.
(e) (CH3)3C–O– + CH3CH2OH ⇌
Problem 42f
Complete the following acid–base reactions. In each case, indicate whether the equilibrium favors the reactants or the products, and explain your reasoning.
(f) (CH3)3C–O– + H2O ⇌
Problem 42g
Complete the following acid–base reactions. In each case, indicate whether the equilibrium favors the reactants or the products, and explain your reasoning.
(g) KOH + CH3CH2OH ⇌
Problem 43a
Suggest carbonyl compounds and reducing agents that might be used to form the following alcohols.
(a) octan-1-ol
Problem 43b
Suggest carbonyl compounds and reducing agents that might be used to form the following alcohols.
(b) 1-cyclohexylpropan-1-ol
Problem 43c,d
Suggest carbonyl compounds and reducing agents that might be used to form the following alcohols.
(c) 1-phenylbutan-1-ol
(d)
Problem 43e,f
Suggest carbonyl compounds and reducing agents that might be used to form the following alcohols.
(e)
(f)
Problem 44a
Show how you would synthesize the following compounds from any starting materials containing no more than six carbon atoms.
(a)
Problem 44b
Show how you would synthesize the following compounds from any starting materials containing no more than six carbon atoms.
(b)
Problem 45
Geminal diols, or 1,1-diols, are usually unstable, spontaneously losing water to give carbonyl compounds. Therefore, geminal diols are regarded as hydrated forms of ketones and aldehydes. Propose a mechanism for the acid-catalyzed loss of water from propane-2,2-diol to give acetone.
Problem 46a
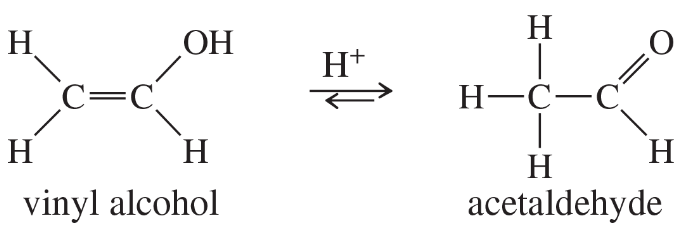
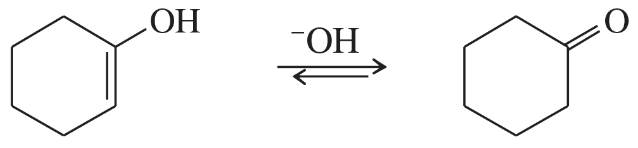
Vinyl alcohols are generally unstable, quickly isomerizing to carbonyl compounds. Propose mechanisms for the following isomerizations.
(a)
Problem 46b
Vinyl alcohols are generally unstable, quickly isomerizing to carbonyl compounds. Propose mechanisms for the following isomerizations.
(b)
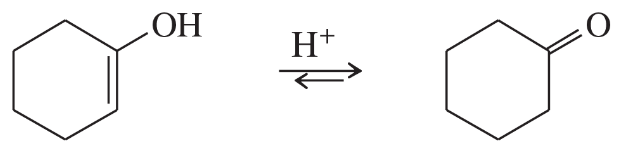
Problem 46c
Vinyl alcohols are generally unstable, quickly isomerizing to carbonyl compounds. Propose mechanisms for the following isomerizations.
(c)
Problem 47
Compound A (C7H11Br) is treated with magnesium in ether to give B (C7H11MgBr), which reacts violently with D2O to give 1-methylcyclohexene with a deuterium atom on the methyl group (C). Reaction of B with acetone (CH3COCH3) followed by hydrolysis gives D (C10H18O). Heating D with concentrated H2SO4 gives E (C10H16), which decolorizes two equivalents of Br2 to give F (C10H16Br4). E undergoes hydrogenation with excess H2 and a Pt catalyst to give isobutylcyclohexane. Determine the structures of compounds A through F, and show your reasoning throughout.
Problem 48
Grignard reagents react slowly with oxetane to produce primary alcohols. Propose a mechanism for this reaction, and suggest why oxetane reacts with Grignard reagents even though most ethers do not.
Problem 49
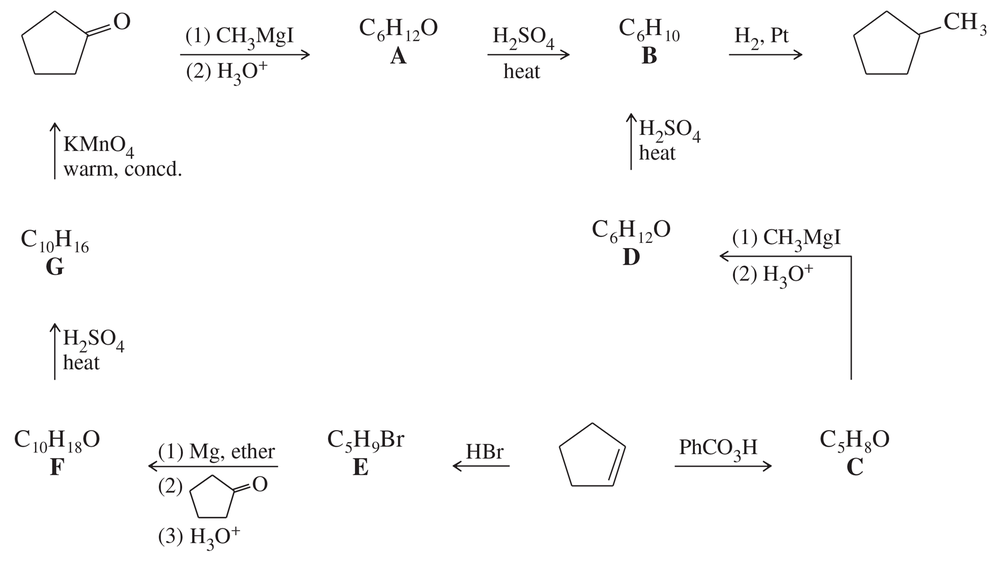
Determine the structures of compounds A through G, including stereochemistry where appropriate.
Problem 50
Many hunting dogs enjoy standing nose-to-nose with a skunk while barking furiously, oblivious to the skunk spray directed toward them. One moderately effective way of lessening the amount of odor is to wash the dog in a bath containing dilute hydrogen peroxide, sodium bicarbonate, and some mild dish detergent. Use chemical reactions to describe how this mixture helps to remove the skunk spray from the dog. The two major components of skunk oil are 3-methylbutane-1-thiol and but-2-ene-1-thiol.
Problem 51a-d
Propose structures for intermediates and products (A) through (D).
Problem 51a-d
Propose structures for intermediates and products (A) through (D)
Problem 51f,g
Propose structures for intermediates and products (F) through (G)
Problem 52a
Devise a synthesis for each compound, starting with methylenecyclohexane and any other reagents you need.
a. 1-methylcyclohexanol
Problem 52b
Devise a synthesis for each compound, starting with methylenecyclohexane and any other reagents you need.
b. cyclohexylmethanol
Problem 52c
Devise a synthesis for each compound, starting with methylenecyclohexane and any other reagents you need.
c. 1-(hydroxymethyl)cyclohexanol