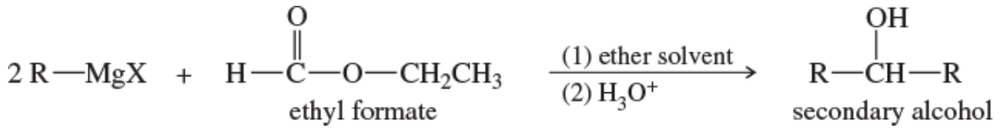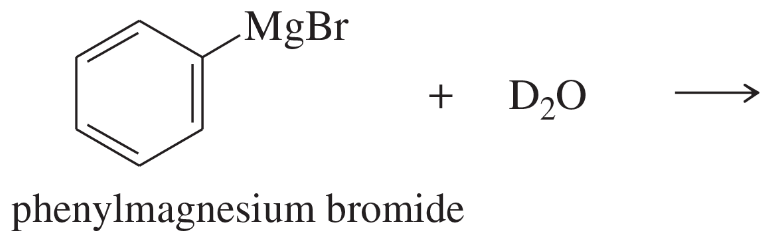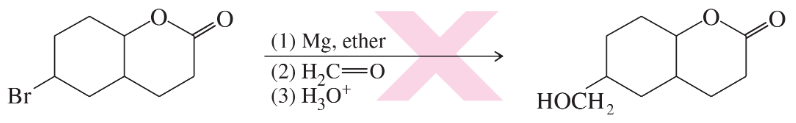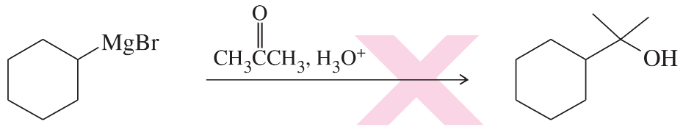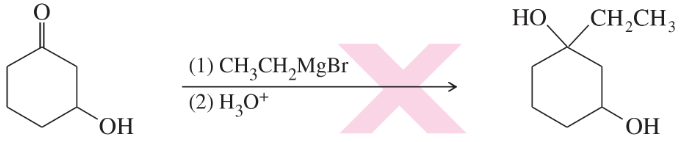Back
BackProblem 18a
A formate ester, such as ethyl formate, reacts with an excess of a Grignard reagent to give (after protonation) secondary alcohols with two identical alkyl groups.
(a) Propose a mechanism to show how the reaction of ethyl formate with an excess of allylmagnesium bromide gives, after protonation, hepta-1,6-dien-4-ol.
Problem 18b
A formate ester, such as ethyl formate, reacts with an excess of a Grignard reagent to give (after protonation) secondary alcohols with two identical alkyl groups.
(b) Show how you would use reactions of Grignard reagents with ethyl formate to synthesize the following secondary alcohols.
(i) pentan-3-ol
(ii) diphenylmethanol
(iii) trans,trans-nona-2,7-dien-5-ol
Problem 19a
Show how you would synthesize the following alcohol by adding Grignard reagents to ethylene oxide.
(a) 2-phenylethanol
Problem 19c
Show how you would synthesize the following alcohol by adding Grignard reagents to ethylene oxide.
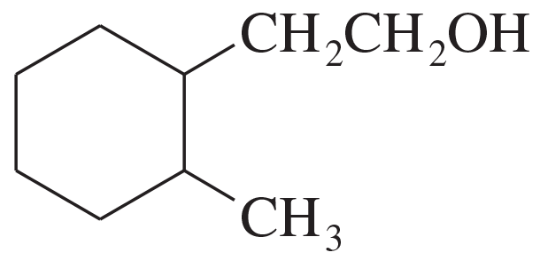
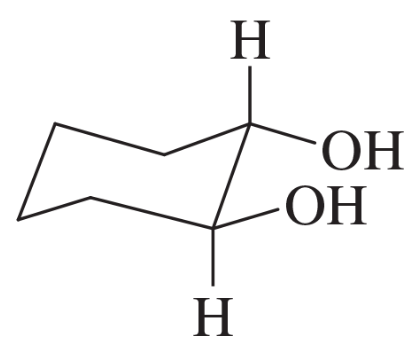
(c)
Problem 20a,b
Acetylide ions also add to ethylene oxide much like Grignard and organolithium reagents. Predict the products obtained by adding the following acetylide ions to ethylene oxide, followed by a dilute acid workup.
(a) HC≡C:–
(b) CH3CH2–C≡C:–
Problem 21a
Show how you would synthesize the following compound from alkyl halides, vinyl halides, and aryl halides containing no more than six carbon atoms.
(a) octane
Problem 21c
Show how you would synthesize the following compound from alkyl halides, vinyl halides, and aryl halides containing no more than six carbon atoms.
(c) trans-oct-3-ene
Problem 21d
Show how you would synthesize the following compound from alkyl halides, vinyl halides, and aryl halides containing no more than six carbon atoms.
(d) cyclopentyl propyl ketone
Problem 22a,b,c
Predict the products of the following reactions.
(a) sec-butylmagnesium iodide + D2O
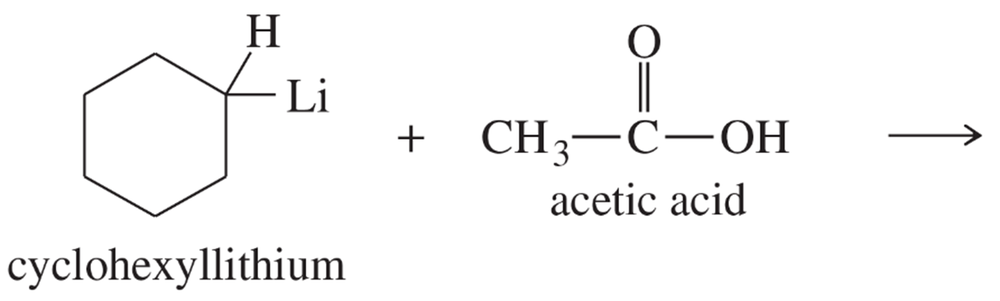
(b) n-butyllithium + CH3CH2OH
(c) isobutylmagnesium bromide + but-1-yne
Problem 22d,e
Predict the products of the following reactions.
(d)
(e)
Problem 23a
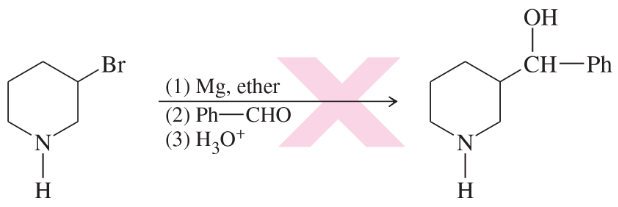
Point out the flaws in the following incorrect Grignard syntheses.
(a)
Problem 23b
Point out the flaws in the following incorrect Grignard syntheses.
(b)
Problem 23c,d
Point out the flaws in the following incorrect Grignard syntheses.
(c)
(d)
Problem 24a
Predict the products you would expect from the reaction of NaBH4 with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(a) CH3–(CH2)8–CHO
Problem 24c
Predict the products you would expect from the reaction of NaBH4 with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(c) Ph-COOH
Problem 24d
Predict the products you would expect from the reaction of NaBH4 with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(d)
Problem 24e
Predict the products you would expect from the reaction of NaBH4 with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(e)
Problem 24f
Predict the products you would expect from the reaction of NaBH4 with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(f)
Problem 25a
Predict the products you would expect from the reaction of LiAlH4 followed by hydrolysis with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(a) CH3–(CH2)8–CHO
Problem 25c
Predict the products you would expect from the reaction of LiALH4 followed by hydrolysis with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(c) Ph-COOH
Problem 25d
Predict the products you would expect from the reaction of LiAlH4 followed by hydrolysis with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(d)
Problem 25e
Predict the products you would expect from the reaction of LiAlH4 followed by hydrolysis with the following compounds. You may assume that these reactions take place in methanol as the solvent.
(e)
Problem 26a
Show how you would synthesize the following alcohol by reducing appropriate carbonyl compound.
a. heptan-1-ol
Problem 26c
Show how you would synthesize the following alcohol by reducing appropriate carbonyl compound.
c. 2-methylhexan-3-ol
Problem 26d
Show how you would synthesize the following alcohol by reducing appropriate carbonyl compound.
(d)
Problem 27
Arrange the following compounds in order of decreasing acidity.
CH3COOH, CH3OH, CH3CH3, CH3SO3H, CH3NH2, CH3SH, CH3C≡CH
Problem 28
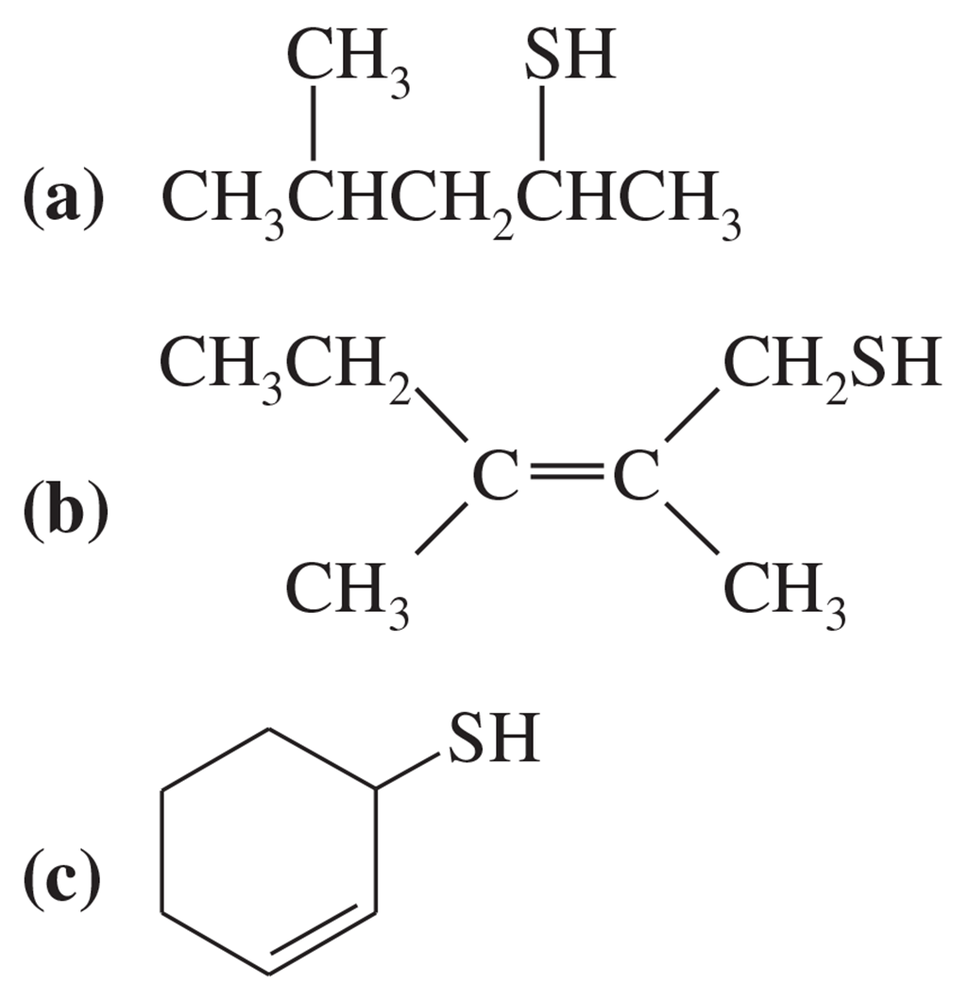
Give IUPAC names for the following compounds.
Problem 29
Authentic skunk spray has become valuable for use in scent-masking products. Show how you would synthesize the major component of skunk spray (3-methylbutane-1-thiol and but-2-ene-1-thiol) from any of the readily available butene or from buta-1,3-diene.
Problem 30f,g
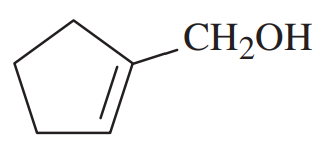
Give a systematic (IUPAC) name for each alcohol. Classify each as primary, secondary, or tertiary.
(f)
(g)
Problem 31a,b
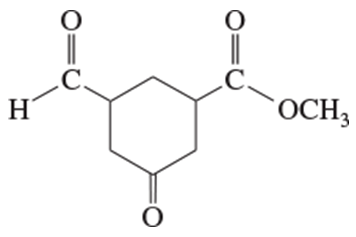
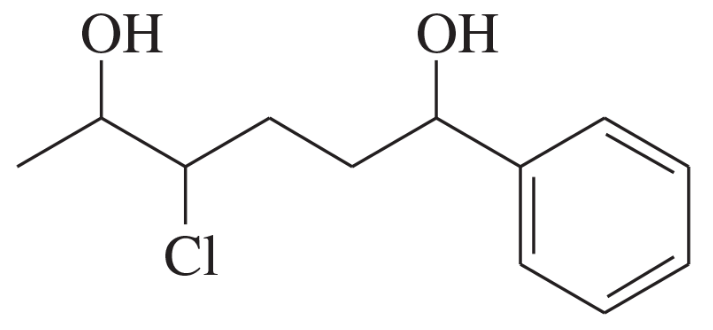
Give systematic (IUPAC) names for the following diols and phenols.
(a)
(b)