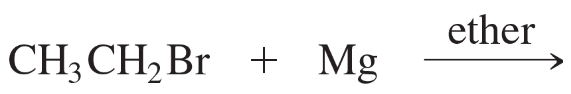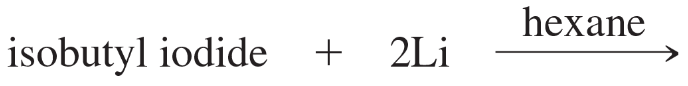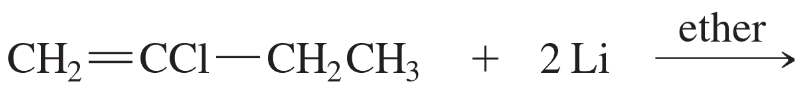Back
BackProblem 2
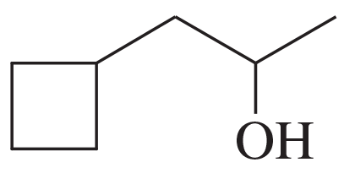
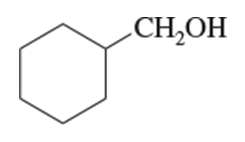
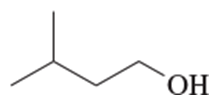
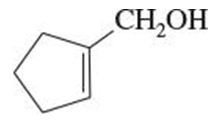
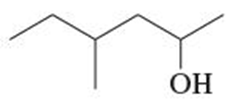
Give both the IUPAC name and the common name for each alcohol.
(a) CH3CH2CH(OH)CH3
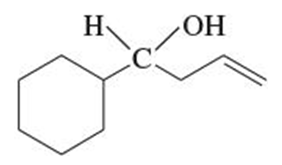
(b)
(c)
(d) (CH3)2CHCH2CH2OH
Problem 3a
For each molecular formula, draw all the possible constitutional isomers of alcohols with that formula. Give the IUPAC name for each alcohol.
(a) C3H8O
Problem 3b
For each molecular formula, draw all the possible constitutional isomers of alcohols with that formula. Give the IUPAC name for each alcohol.
(b) C4H10O
Problem 3c
For each molecular formula, draw all the possible constitutional isomers of alcohols with that formula. Give the IUPAC name for each alcohol.
(c) C3H6O
Problem 3d
For each molecular formula, draw all the possible constitutional isomers of alcohols with that formula. Give the IUPAC name for each alcohol.
(d) C3H4O
Problem 4a,b,c
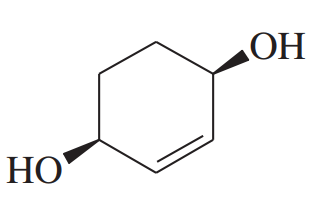
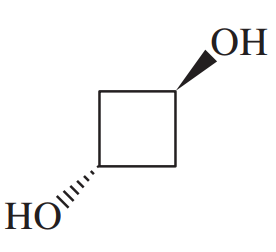
Give a systematic (IUPAC) name for each diol
(a) CH3CH(OH)(CH2)4CH(OH)C(CH3)3
(b) HO-(CH2)8-OH
(c)
Problem 4d,e
Give a systematic (IUPAC) name for each diol
(d)
(e)
Problem 5
Predict which member of each pair will be more soluble in water. Explain the reasons for your answers.
(a) hexan-1-ol or cyclohexanol
(b) heptan-1-ol or 4-methylphenol
(c) 3-ethylhexan-3-ol or octan-2-ol
(d) hexan-2-ol or cyclooctane-1,4-diol
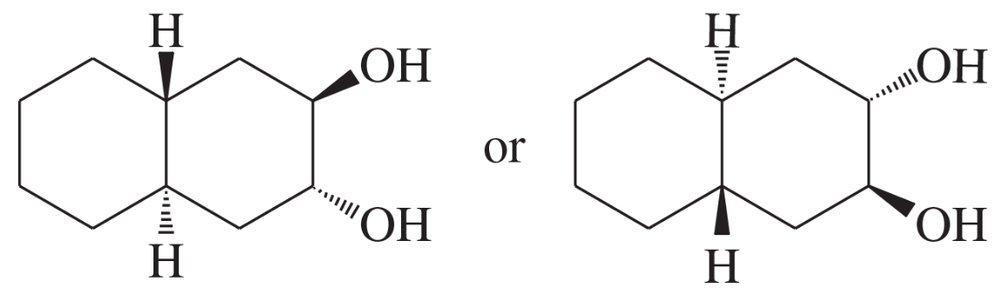
(e)
Problem 6
Dimethylamine, (CH3)2NH, has a molecular weight of 45 and a boiling point of 7.4 °C. Trimethylamine, (CH3)3N, has a higher molecular weight (59) but a lower boiling point (3.5 °C). Explain this apparent discrepancy.
Problem 7c
Predict which member of each pair will be more acidic. Explain your answers.
c. 2-chloroethanol or 2,2-dichloroethanol
Problem 7d
Predict which member of each pair will be more acidic. Explain your answers.
d. 2,2-dichloropropan-1-ol or 2,2-difluoropropan-1-ol
Problem 8
Without looking them up, rank the following compounds in decreasing order of acidity. These examples represent large classes of compounds that differ widely in acidity.
water, ethanol, 2-chloroethanol, tert-butyl alcohol, ammonia, sulfuric acid, hexane, hex-1-yne, acetic acid
Problem 9
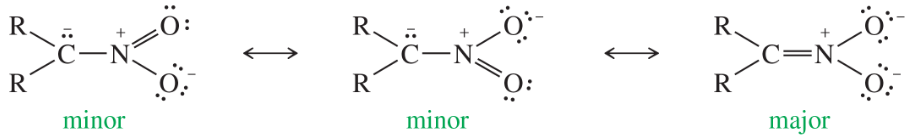
A nitro group (–NO2) effectively stabilizes a negative charge on an adjacent carbon atom through resonance:
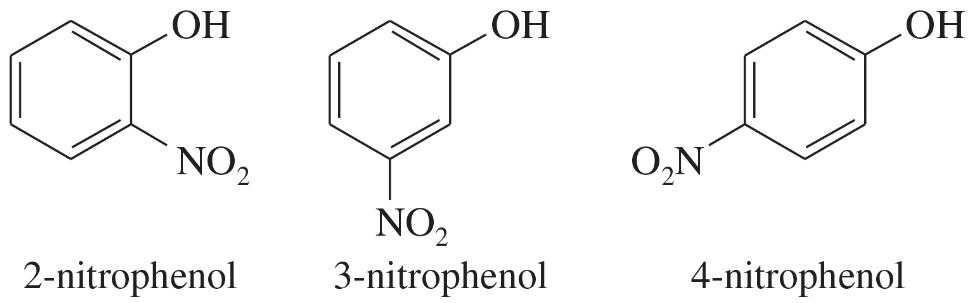
Two of the following nitrophenols are much more acidic than phenol itself. The third compound is only slightly more acidic than phenol. Use resonance structures of the appropriate phenoxide ions to show why two of these anions should be unusually stable.
- For each molecular formula, draw all the possible constitutional isomers of alcohols with that formula. Give the IUPAC name for each alcohol. (a) C3H8O (b) C4H10O (c) C3H6O (d) C3H4O
Problem 10
Problem 10a
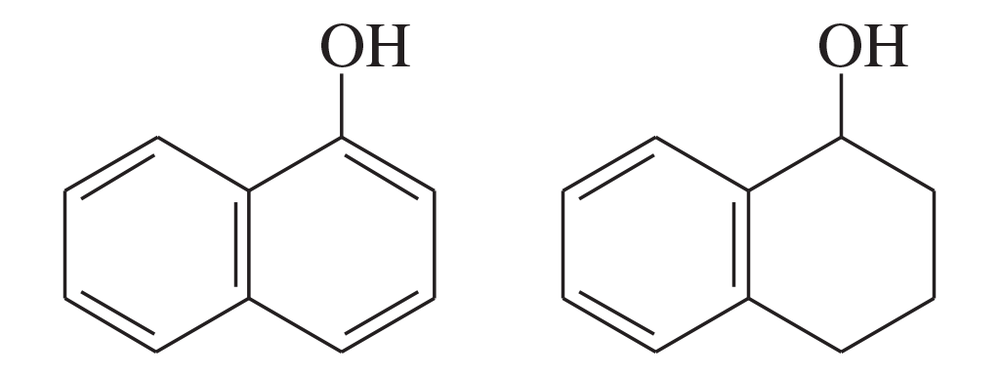
The following compounds are only slightly soluble in water, but one of them is very soluble in a dilute aqueous solution of sodium hydroxide. The other is still only slightly soluble.
(a) Explain the difference in solubility of these compounds in dilute sodium hydroxide.
Problem 10b
The following compounds are only slightly soluble in water, but one of them is very soluble in a dilute aqueous solution of sodium hydroxide. The other is still only slightly soluble.
(b) Show how this difference might be exploited to separate a mixture of these two compounds using a separatory funnel.
Problem 11a-h
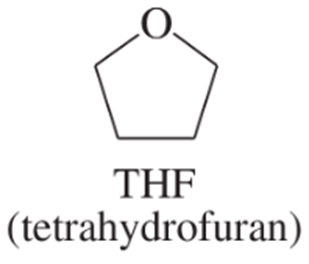
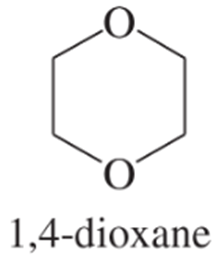
Which of the following compounds are suitable solvents for Grignard reactions?
(a) n-hexane
(b) CH3–O–CH3
(c) CHCl3
(d) cyclohexane
(e) benzene
(f) CH3OCH2CH2OCH3
(g)
(h)
Problem 12a,b
Predict the products of the following reactions.
(a)
(b)
Problem 12c,d
Predict the products of the following reactions.
(c)
(d)
Problem 13a
Show how you would synthesize the following primary alcohol by adding an appropriate Grignard reagent to formaldehyde.
(a)
Problem 13b
Show how you would synthesize the following primary alcohol by adding an appropriate Grignard reagent to formaldehyde.
(b)
Problem 13c
Show how you would synthesize the following primary alcohol by adding an appropriate Grignard reagent to formaldehyde.
(c)
Problem 14a
Show two ways you could synthesize each of the following secondary alcohol by adding an appropriate Grignard reagent to an aldehyde.
(a)
Problem 14c
Show two ways you could synthesize each of the following secondary alcohol by adding an appropriate Grignard reagent to an aldehyde.
(c)
Problem 15b
Show how you would synthesize following tertiary alcohol by adding an appropriate Grignard reagent to a ketone.
b. Ph3COH
Problem 15c
Show how you would synthesize following tertiary alcohol by adding an appropriate Grignard reagent to a ketone.
c. 1-ethylcyclopentanol
Problem 15d
Show how you would synthesize following tertiary alcohol by adding an appropriate Grignard reagent to a ketone.
d. 2-cyclopentylpentan-2-ol
Problem 16
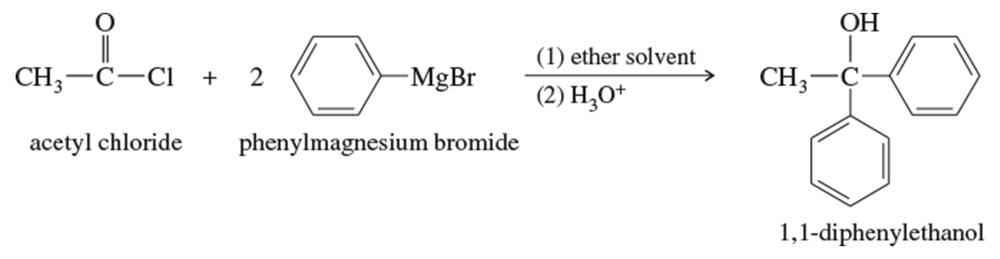
Propose a mechanism for the reaction of acetyl chloride with phenylmagnesium bromide to give 1,1-diphenylethanol.
Problem 17a
Show how you would add Grignard reagent to acid chloride or ester to synthesize the following alcohols.
a. Ph3C–OH
Problem 17c
Show how you would add Grignard reagent to acid chloride or ester to synthesize the following alcohols.
c. dicyclohexylphenylmethanol