Back
BackProblem 52d
Devise a synthesis for each compound, starting with methylenecyclohexane and any other reagents you need.
d. trans-2-methylcyclohexanol
Problem 52f
Devise a synthesis for each compound, starting with methylenecyclohexane and any other reagents you need.
f. 1-(phenylmethyl)cyclohexanol
Problem 53a
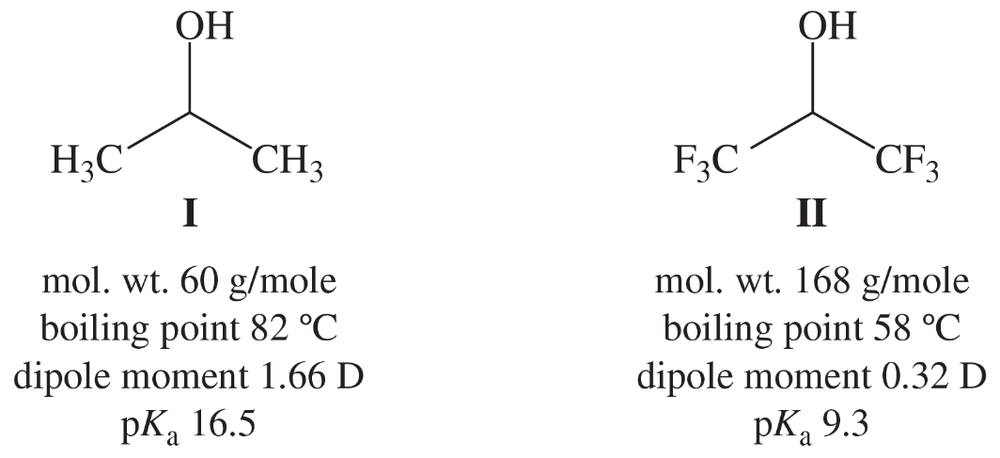
Compare the properties of propan-2-ol (I) and the hexafluoro analog (II).
(a) Compound II has almost triple the molecular weight of I, but II has a lower boiling point. Explain.
Problem 54a
Compounds containing deuterium (D = 2H) are useful for kinetic studies and metabolic studies with new pharmaceuticals. One way to introduce deuterium is by using the reagent LiAlD4, equivalent in reactivity to LiAlH4. Show how to make these deuterium-labeled compounds, using LiAlD4 and D2O as your sources of deuterium, and any non-deuterated starting materials you wish.
a. CH3CHDOH
Problem 54b
Compounds containing deuterium (D = 2H) are useful for kinetic studies and metabolic studies with new pharmaceuticals. One way to introduce deuterium is by using the reagent LiAlD4, equivalent in reactivity to LiAlH4. Show how to make these deuterium-labeled compounds, using LiAlD4 and D2O as your sources of deuterium, and any non-deuterated starting materials you wish.
(b) CH3CD2OH
Problem 54c
Compounds containing deuterium (D = 2H) are useful for kinetic studies and metabolic studies with new pharmaceuticals. One way to introduce deuterium is by using the reagent LiAlD4, equivalent in reactivity to LiAlH4. Show how to make these deuterium-labeled compounds, using LiAlD4 and D2O as your sources of deuterium, and any non-deuterated starting materials you wish.
(c) CH3CD2OD
Problem 55a
Show how to make these deuterium-labeled compounds, using CD3MgBr and D2O as your sources of deuterium, and any non-deuterated starting materials you wish.
a. CH3CH(OD)CD3
Problem 55b
Show how to make these deuterium-labeled compounds, using CD3MgBr and D2O as your sources of deuterium, and any non-deuterated starting materials you wish.
b. CH3C(OH)(CD3)2
Problem 55c
Show how to make these deuterium-labeled compounds, using CD3MgBr and D2O as your sources of deuterium, and any non-deuterated starting materials you wish.
c. CD3CH2CH2OH
Problem 55d
Show how to make these deuterium-labeled compounds, using CD3MgBr and D2O as your sources of deuterium, and any non-deuterated starting materials you wish.
d. Ph(CD3)2COD
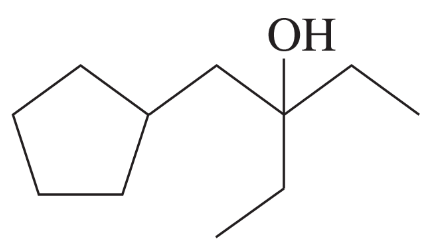
Problem 56a
Often, compounds can be synthesized by more than one method. Show how this 3° alcohol can be made from the following:
(a) two different ketones
Problem 56b
Often, compounds can be synthesized by more than one method. Show how this 3° alcohol can be made from the following:
(b) two different alkenes
Problem 56c
Often, compounds can be synthesized by more than one method. Show how this 3° alcohol can be made from the following:
(c) an ester
Problem 56d
Often, compounds can be synthesized by more than one method. Show how this 3° alcohol can be made from the following:
(d) a 3° alkyl bromide
Problem 57a
Show how this 1° alcohol can be made from the following:
(a) a 1° alkyl bromide
Problem 57b
Show how this 1° alcohol can be made from the following:
(b) formaldehyde
Problem 57c,d
Show how this 1° alcohol can be made from the following:
(c) a 7-carbon aldehyde
(d) a carboxylic acid
Problem 57e,f
Show how this 1° alcohol can be made from the following:
(e) an alkene
(f) ethylene oxide
Problem 58a
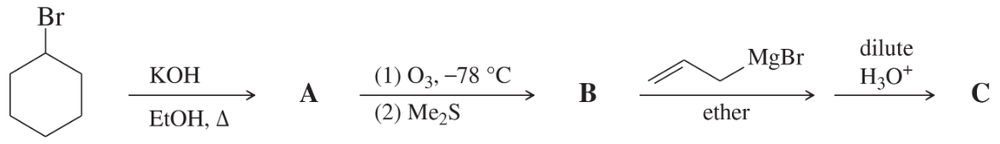
For each synthesis, start with bromocyclohexane and predict the products. Assume that an excess of each reactant is added so that all possible reactions that can happen will happen.
(a)
Problem 58b
For each synthesis, start with bromocyclohexane and predict the products. Assume that an excess of each reactant is added so that all possible reactions that can happen will happen.
(b)
Problem 58c
For each synthesis, start with bromocyclohexane and predict the products. Assume that an excess of each reactant is added so that all possible reactions that can happen will happen.
(c)
Problem 60a,b
Problem 8-54 describes a new method to perform ozonolysis reactions that used pyridine (py) to generate the final aldehydes and ketones in a non-aqueous reaction medium. In a subsequent publication (J. Org. Chem., 2013, 78, 42), Professor Dussault (U. of Nebraska at Lincoln) described a “tandem” process in which two reactions are performed sequentially without having to isolate the intermediate aldehyde or ketone. Show the final product from each sequence. (Hint: The isolated products were from the larger part of the structure. Ignore stereochemistry.)
(a)
(b)
Problem 60c,d
Problem 8-54 describes a new method to perform ozonolysis reactions that used pyridine (py) to generate the final aldehydes and ketones in a non-aqueous reaction medium. In a subsequent publication (J. Org. Chem., 2013, 78, 42), Professor Dussault (U. of Nebraska at Lincoln) described a “tandem” process in which two reactions are performed sequentially without having to isolate the intermediate aldehyde or ketone. Show the final product from each sequence. (Hint: The isolated products were from the larger part of the structure. Ignore stereochemistry.)
(c)
(d)