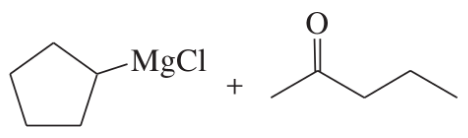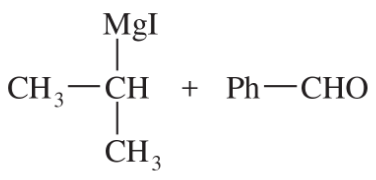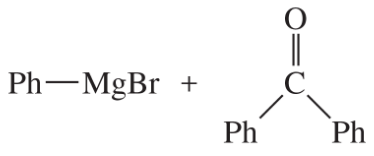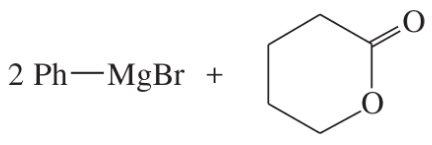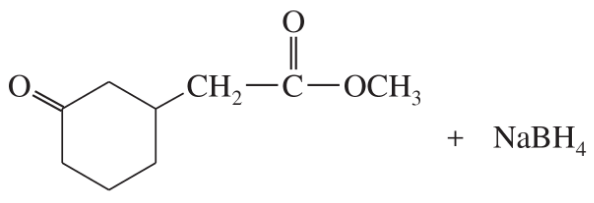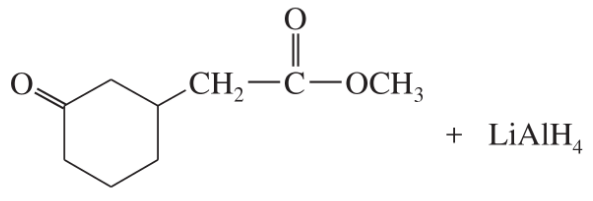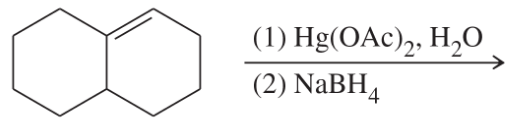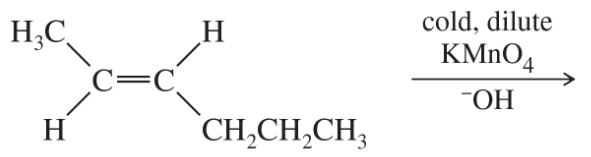Back
BackProblem 32a,b,c
Draw the structures of the following compounds. (Includes both new and old names.)
(a) triphenylmethanol
(b) 4-(chloromethyl)heptan-3-ol
(c) 2-cyclohexen-1-ol
Problem 32d,e
Draw the structures of the following compounds. (Includes both new and old names.)
(d) 3-cyclopentylhexan-3-ol
(e) meso-2,4-pentanediol
Problem 32i,j
Draw the structures of the following compounds. (Includes both new and old names.)
i. cyclopent-3-ene-1-thiol
j. dimethyl disulfide
Problem 32k
Draw the structures of the following compounds. (Includes both new and old names.)
(k) 3-methylhex-4-yn-2-ol
Problem 33a,b
Predict which member of each pair has the higher boiling point, and explain the reasons for your predictions.
a. hexan-1-ol or 3,3-dimethylbutan-1-ol
b. hexan-2-one or hexan-2-ol
Problem 34a
Predict which member of each pair is more acidic, and explain the reasons for your predictions.
a. cyclopentanol or 3-chlorophenol
Problem 34b
Predict which member of each pair is more acidic, and explain the reasons for your predictions.
b. cyclohexanol or cyclohexanethiol
Problem 35a
Predict which member of each group is most soluble in water, and explain the reasons for your predictions.
a. butan-1-ol, pentan-1-ol, or propan-2-ol
Problem 35b
Predict which member of each group is most soluble in water, and explain the reasons for your predictions.
b. chlorocyclohexane, cyclohexanol, or cyclohexane-1,2-diol
Problem 36a,b
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis).
(a)
(b)
Problem 36c,d
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis).
(c)
(d)
Problem 36f,g
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis).
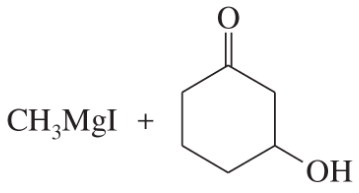
(f)
(g)
Problem 36j
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis).
(j)
Problem 36k,l
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis).
(k)
(l)
Problem 36m
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis).
(m)
Problem 36n
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis).
(n)
Problem 36p
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis).
(p)
Problem 37a
Starting from bromobenzene and any other reagents and solvents you need, show how you would synthesize the following compounds. Any of these products may be used as starting materials in subsequent parts of this problem.
a. 1-phenylpropan-1-ol
Problem 37b
Starting from bromobenzene and any other reagents and solvents you need, show how you would synthesize the following compounds. Any of these products may be used as starting materials in subsequent parts of this problem.
b. 1-phenylpropene
Problem 37c
Starting from bromobenzene and any other reagents and solvents you need, show how you would synthesize the following compounds. Any of these products may be used as starting materials in subsequent parts of this problem.
c. 1-phenylpropan-2-ol
Problem 37d
Starting from bromobenzene and any other reagents and solvents you need, show how you would synthesize the following compounds. Any of these products may be used as starting materials in subsequent parts of this problem.
d. 3-phenylprop-2-en-1-ol
Problem 38
Write structures for a homologous series of alcohols (R―OH) having from one to six carbons.
Problem 38a
Show how you would synthesize the following alcohol from appropriate alkene.
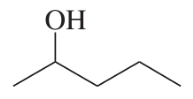
(a)
Problem 38b
Show how you would synthesize the following alcohol from appropriate alkene.
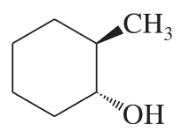
(b)
Problem 38d
Show how you would synthesize the following alcohol from appropriate alkene.
(d)
Problem 39a
Show how you would use Grignard syntheses to prepare the following alcohol from the indicated starting material and any other necessary reagents.
(a) octan-3-ol from hexanal, CH3(CH2)4CHO
Problem 39d
Show how you would use Grignard syntheses to prepare the following alcohol from the indicated starting material and any other necessary reagents.
(d) 2-cyclohexylethanol from bromocyclohexane
Problem 39e
Show how you would use Grignard syntheses to prepare the following alcohol from the indicated starting material and any other necessary reagents.
(e) benzyl alcohol (Ph–CH2–OH) from bromobenzene (Ph–Br)
Problem 39g
Show how you would use Grignard syntheses to prepare the following alcohol from the indicated starting material and any other necessary reagents.
(g) cyclopentylphenylmethanol from benzaldehyde (Ph–CHO)
Problem 40b
Show how you would accomplish the following transformations. You may use any additional reagents you need.
(b)