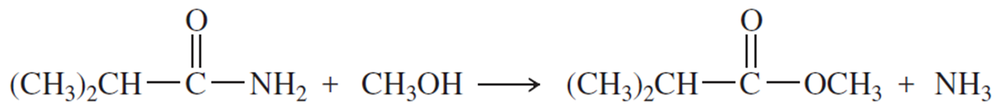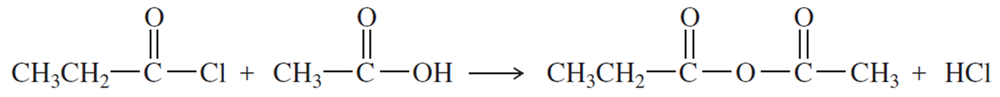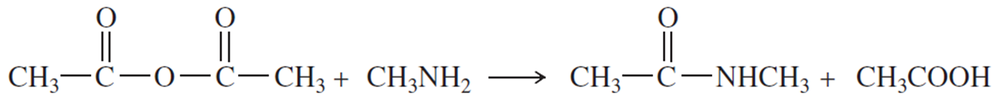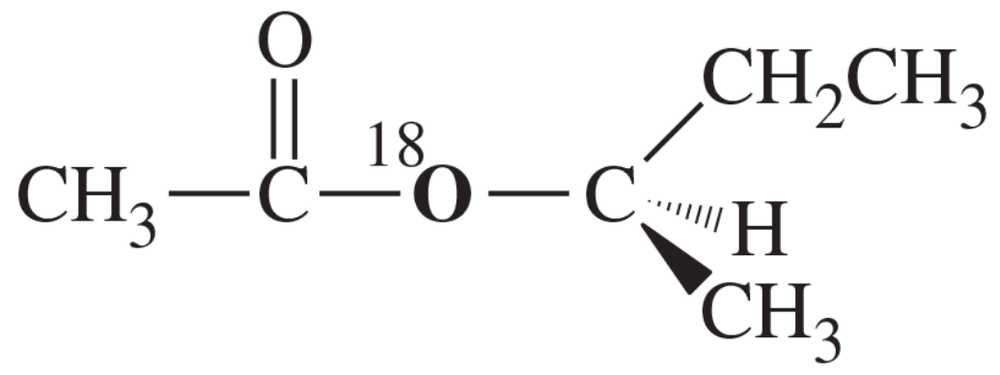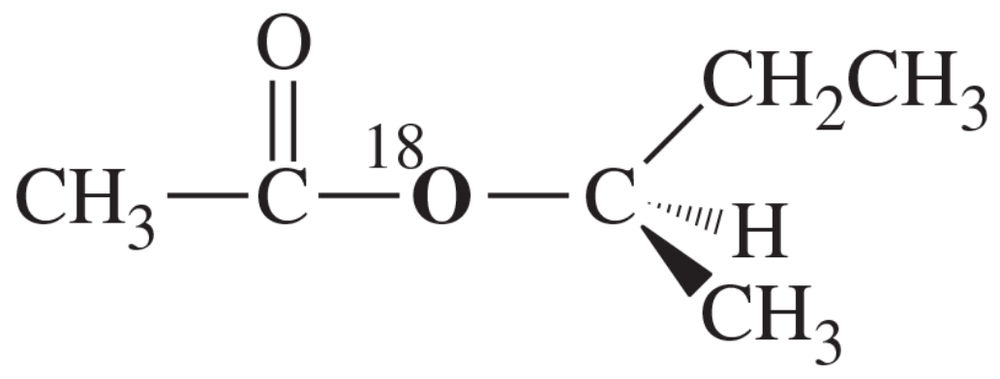Back
BackProblem 1a,b,c
Name the following carboxylic acid derivatives, giving both a common name and an IUPAC name where possible.
(a) PhCOOCH2CH(CH3)2
(b) PhOCHO
(c) PhCH(CH3)COOCH3
Problem 1d,e
Name the following carboxylic acid derivatives, giving both a common name and an IUPAC name where possible.
(d) PhNHCOCH2CH(CH3)2
(e) CH3CONHCH2Ph
Problem 1f
Name the following carboxylic acid derivatives, giving both a common name and an IUPAC name where possible.
(f) CH3CH(OH)CH2CN
Problem 1m,n
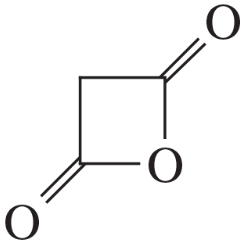
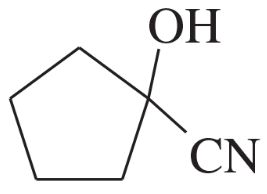
Name the following carboxylic acid derivatives, giving both a common name and an IUPAC name where possible.
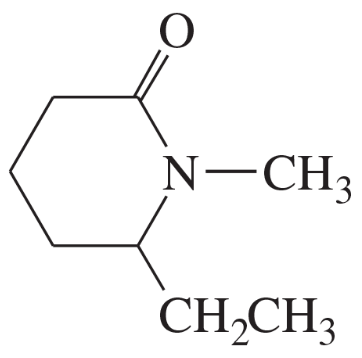
(m)
(n)
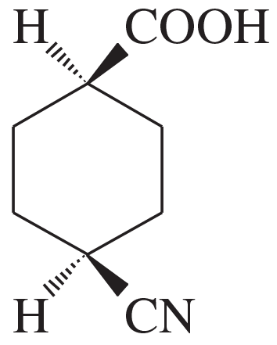
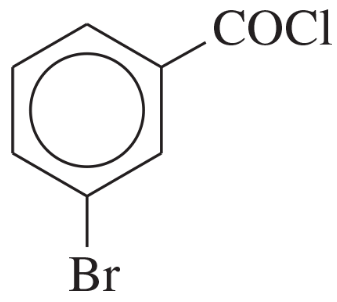
Problem 1o,p
Name the following carboxylic acid derivatives, giving both a common name and an IUPAC name where possible.
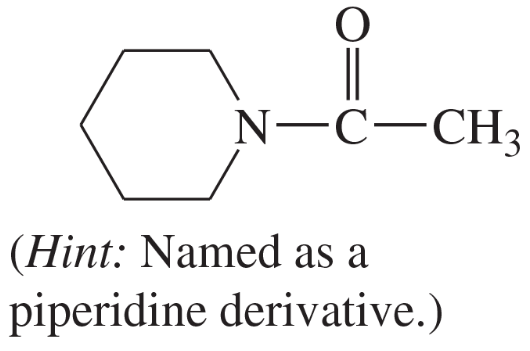
(o)
(p)
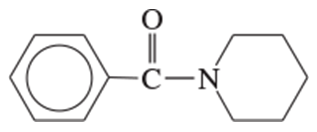
Problem 1q,r
Name the following carboxylic acid derivatives, giving both a common name and an IUPAC name where possible.
(q)
(r)
Problem 6a
Propose a mechanism for the reaction of benzyl alcohol with acetyl chloride to give benzyl acetate.
Problem 6b
Propose a mechanism for the reaction of benzoic acid with acetyl chloride to give acetic benzoic anhydride.
Problem 6c
Propose a second mechanism for the reaction of benzoic acid with acetyl chloride to give acetic benzoic anhydride. This time, let the other oxygen of benzoic acid serve as the nucleophile to attack the carbonyl group of acetyl chloride. Because proton transfers are fast between these oxygen atoms, it is difficult to differentiate between these two mechanisms experimentally.
Problem 6d
Propose a mechanism for the reaction of aniline with acetic anhydride to give acetanilide.
Problem 6e
Propose a mechanism for the reaction of aniline with ethyl acetate to give acetanilide. What is the leaving group in your proposed mechanism? Would this be a suitable leaving group for an SN2 reaction?
Problem 7
Which of the following proposed reactions would take place quickly under mild conditions?
(a)
(b)
(c)
(d)
(e)
Problem 8a,b
Show how you would synthesize the following esters from appropriate acyl chlorides and alcohols.
(a) ethyl propionate
(b) phenyl 3-methylhexanoate
Problem 8c,d
Show how you would synthesize the following esters from appropriate acyl chlorides and alcohols.
(c) benzyl benzoate
(d) cyclopropyl cyclohexanecarboxylate
Problem 8e,f
Show how you would synthesize the following esters from appropriate acyl chlorides and alcohols.
(e) tert-butyl acetate
(f) diallyl succinate
Problem 9a,b
Show how you would use appropriate acyl chlorides and amines to synthesize the following amides.
(a) N,N-dimethylacetamide
(b) acetanilide (PhNHCOCH3)
Problem 9c,d
Show how you would use appropriate acyl chlorides and amines to synthesize the following amides.
(c) cyclohexanecarboxamide
(d)
Problem 10a
Show how you would use acetic anhydride and an appropriate alcohol or amine to synthesize (i) benzyl acetate and (ii) N,N-diethylacetamide.
Problem 11
Propose a mechanism for the reaction of benzyl acetate with methylamine. Label the attacking nucleophile and the leaving group, and draw the transition state in which the leaving group leaves.
Problem 12
When ethyl 4-hydroxybutyrate is heated in the presence of a trace of a basic catalyst (sodium acetate), one of the products is a lactone. Propose a mechanism for formation of this lactone.
Problem 13
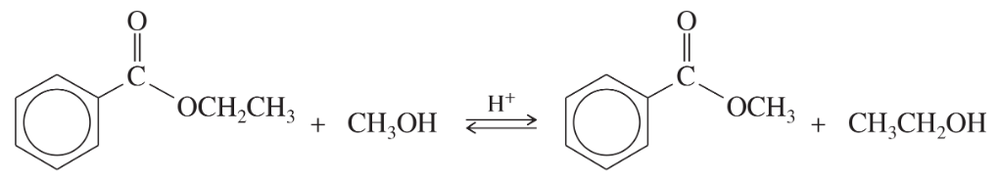
Acid-catalyzed transesterification:
Complete the mechanism for this acid-catalyzed transesterification by drawing out all the individual steps. Draw the important resonance contributors for each resonance-stabilized intermediate.
Problem 14
Propose a mechanism for the acid-catalyzed hydrolysis of phenylalanine ethyl ester.
Problem 14a
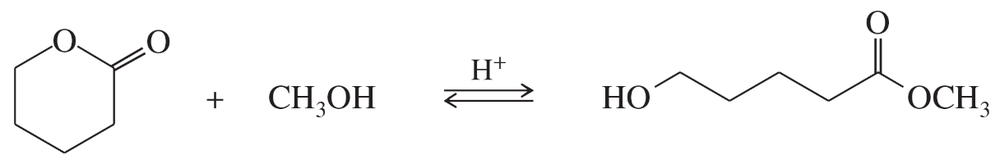
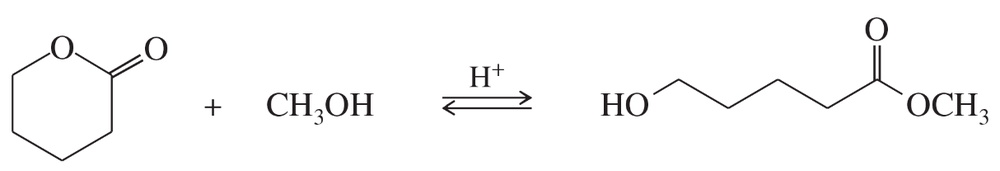
Propose a mechanism for the following ring-opening transesterification. Use the mechanism in Problem 21-13 as a model.
Problem 15
1. Propose a mechanism for the acid-catalyzed reaction of salicylic acid with acetic anhydride.
2. Explain why a single drop of sulfuric acid dramatically increases the reaction rate.
Problem 16a
Suppose we have some optically pure (R)-2-butyl acetate that has been 'labeled' with the heavy 18O isotope at one oxygen atom as shown.
(a) Draw a mechanism for the hydrolysis of this compound under basic conditions. Predict which of the products will contain the 18O label. Also predict whether the butan-2-ol product will be pure (R), pure (S), or racemized.
Problem 16b
Suppose we have some optically pure (R)-2-butyl acetate that has been "labeled" with the heavy 18O isotope at one oxygen atom as shown.
(b) Repeat part (a) for the acid-catalyzed hydrolysis of this compound.
Problem 16c
Suppose we have some optically pure (R)-2-butyl acetate that has been 'labeled' with the heavy 18O isotope at one oxygen atom as shown.
(c) Explain how you would prove experimentally where the 18O label appears in the products. (18O is not radioactive.)
Problem 17a
Explain why we speak of acidic hydrolysis of an ester as acid-catalyzed, but of basic hydrolysis as base-promoted.
Problem 17b
Soap manufacturers always use base to hydrolyze fats, and never acid. Suggest two reasons that basic hydrolysis is preferred.
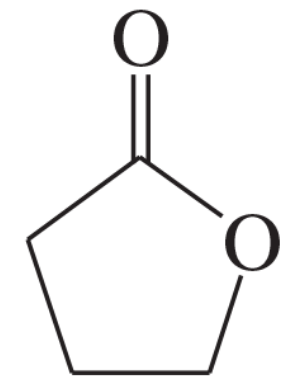
Problem 18
Propose a mechanism for the base-promoted hydrolysis of γ-butyrolactone: