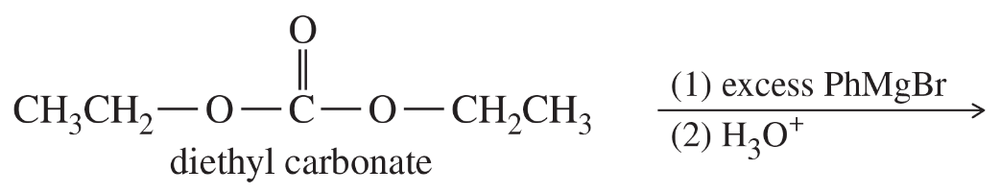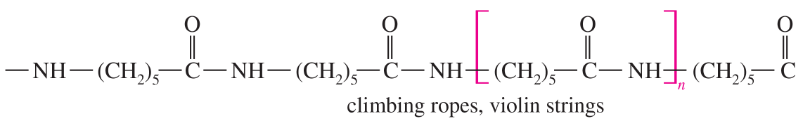Back
BackProblem 54c
Show how you would accomplish the following syntheses. Some of these conversions may require more than one step.
(c) isobutylamine → N-isobutylformamide
Problem 54d
Show how you would accomplish the following syntheses. Some of these conversions may require more than one step.
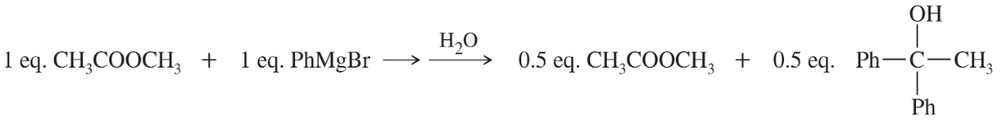
(d) ethyl acetate → 3-methylpentan-3-ol
Problem 54e,f
Show how you would accomplish the following syntheses. Some of these conversions may require more than one step.
(e) cyclohexylamine → N-cyclohexylacetamide
(f) bromocyclohexane → dicyclohexylmethanol
Problem 55a,b
Grignard reagents add to carbonate esters as they add to other esters.
(a) Predict the major product of the following reaction.
(b) Show how you would synthesize 3-ethylpentan-3-ol using diethyl carbonate and ethyl bromide as your only organic reagents
Problem 55c1
Grignard reagents add to carbonate esters as they add to other esters.
(c) Diethyl carbonate is a liquid reagent that is easy to handle. In contrast, phosgene is a highly toxic and corrosive gas. Show how you might use diethyl carbonate instead of phosgene to make Lexan®.
Problem 55c2
Grignard reagents add to carbonate esters as they add to other esters.
(c) Diethyl carbonate is a liquid reagent that is easy to handle. Also show how you might use diethyl carbonate instead of methyl isocyanate to make Sevin® insecticide.
Problem 56
One mole of acetyl chloride is added to a liter of triethylamine, resulting in a vigorous exothermic reaction. Once the reaction mixture has cooled, 1 mole of ethanol is added. Another vigorous exothermic reaction results. The mixture is analyzed and found to contain triethylamine, ethyl acetate, and triethylammonium chloride. Propose mechanisms for the two exothermic reactions.
Problem 57a,b
Show how you would accomplish the following multistep syntheses, using the indicated starting material and any necessary reagents.
(a) hept-6-en-1-ol → ε-caprolactone
(b) methoxybenzene → p-methoxybenzamide
Problem 57c
Show how you would accomplish the following multistep syntheses, using the indicated starting material and any necessary reagents.
(c)
Problem 57d
Show how you would accomplish the following multistep syntheses, using the indicated starting material and any necessary reagents.
(d)
Problem 58a
Methyl p-nitrobenzoate has been found to undergo saponification faster than methyl benzoate.
(a) Consider the mechanism of saponification, and explain the reasons for this rate enhancement.
Problem 58b
Methyl p-nitrobenzoate has been found to undergo saponification faster than methyl benzoate.
(b) Would you expect methyl p-methoxybenzoate to undergo saponification faster or slower than methyl benzoate?
Problem 59a
In each part, rank the compounds in order of increasing rate of nucleophilic attack at C=O by a strong nucleophile like methoxide.
(a)
Problem 59b
In each part, rank the compounds in order of increasing rate of nucleophilic attack at C=O by a strong nucleophile like methoxide.
(b)
Problem 60
Explain this curious result. What does this reaction tell you about the relative reactivity of esters and ketones?
Problem 62a,b
In Section 21-16, we saw that Sevin insecticide is made by the reaction of 1-naphthol with methyl isocyanate. A Union Carbide plant in Bhopal, India, once used this process to make Sevin for use as an agricultural insecticide. On December 3,1984, either by accident or by sabotage, a valve was opened that admitted water to a large tank of methyl isocyanate. The pressure and temperature within the tank rose dramatically, and pressure-relief valves opened to keep the tank from bursting. A large quantity of methyl isocyanate rushed out through the pressure-relief valves, and the vapors flowed with the breeze into populated areas, killing about 2500 people and injuring many more.
(a) Write an equation for the reaction that took place in the tank. Explain why the pressure and temperature rose dramatically.
(b) Propose a mechanism for the reaction you wrote in part (a).
Problem 63a
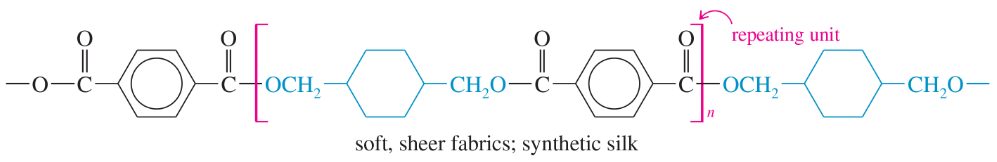
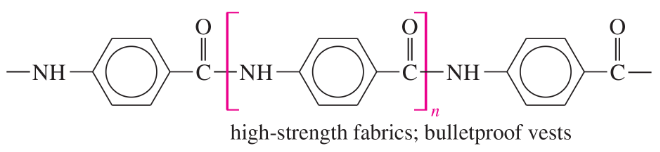
The structures of four useful polymers are shown, together with some of their best-known products. In each case,
(i) Determine the kind of polymer (polyamide, polyester, etc.).
(ii) Draw the structures of the monomers that would be released by complete hydrolysis.
(iii) Suggest what monomers or stable derivatives of the monomers might be used to make these polymers.
(a)
Problem 63b
The structures of four useful polymers are shown, together with some of their best-known products. In each case,
(i) Determine the kind of polymer (polyamide, polyester, etc.).
(ii) Draw the structures of the monomers that would be released by complete hydrolysis.
(iii) Suggest what monomers or stable derivatives of the monomers might be used to make these polymers.
(b)
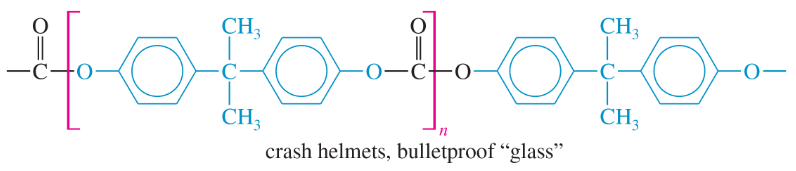
Problem 63c,d(i)
The structures of four useful polymers are shown, together with some of their best-known products. In each case,
(i) determine the kind of polymer (polyamide, polyester, etc.).
(c)
(d)
Problem 66
An unknown compound gives a mass spectrum with a weak molecular ion at m/z 113 and a prominent ion at m/z 68. Its NMR and IR spectra are shown here. Determine the structure, and show how it is consistent with the observed absorptions. Propose a favorable fragmentation to explain the prominent MS peak at m/z 68.
<IMAGE>
Problem 71a,b
Macrolide antibiotics, including erythromycin and azithromycin (Zithromax®), contain a large ring lactone. One of the largest ever reported is Gargantulide A, the structure of which was determined by the research group of Prof. William Gerwick of the Scripps Institution of Oceanography (Organic Letters, 2015, 17, 1377–1380). Isolated from a Streptomyces bacterium, it kills pathogenic bacteria like MRSA and Clostridium difficile, but it proved too toxic to the test animals to continue further testing.
(a) Identify the lactone that makes this a macrolide structure.
(b) How many rings does this structure contain? How many atoms are in the largest ring?