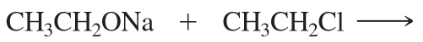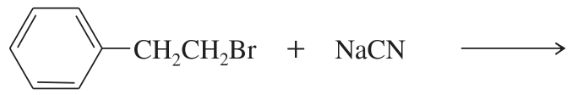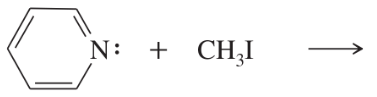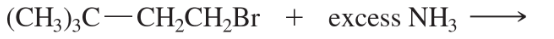Back
BackProblem 43
Two of the carbocations in Problem 6-42 are prone to rearrangement. Show how they might rearrange to more stable carbocations.
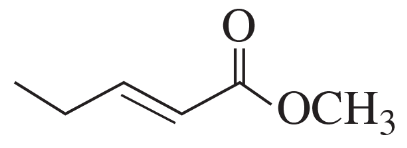
Problem 44b
Draw perspective structures or Fischer projections for the substitution products of the following reactions.
(b)
Problem 45a,b
Predict the products of the following SN2 reactions.
(a)
(b)
Problem 45c,d
Predict the products of the following SN2 reactions.
(c)
(d)
Problem 45e,f
Predict the products of the following SN2 reactions.
(e)
(f)
Problem 46 a
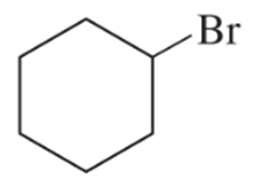
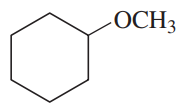
Using cyclohexane as one of your starting materials, show how you would synthesize the following compounds.
(a)
Problem 46c
Using cyclohexane as one of your starting materials, show how you would synthesize the following compounds.
(c)
Problem 46e
Using cyclohexane as one of your starting materials, show how you would synthesize the following compounds.
(e)
Problem 47
Strawberry growers have used large quantities of methyl bromide (b.p. 4 °C) to sterilize the soil before planting their crops. Like some of the freons, methyl bromide can diffuse up into the stratosphere, where it damages the protective ozone layer. Agricultural chemists have suggested using methyl iodide (b.p. 43 °C) as a replacement for methyl bromide. Why is methyl iodide likely to be more toxic to agricultural pests (and people) than methyl bromide? Why is methyl iodide less likely to reach the stratosphere than methyl bromide?
Problem 48a
A solution of pure (S)-2-iodobutane ([α] = +15.90°) in acetone is allowed to react with radioactive iodide, 131I–, until 1.0% of the iodobutane contains radioactive iodine. The specific rotation of this recovered iodobutane is found to be +15.58°.
a. Determine the percentages of (R)- and (S)-2-iodobutane in the product mixture.
Problem 48b
A solution of pure (S)-2-iodobutane ([α] = +15.90°) in acetone is allowed to react with radioactive iodide, 131I–, until 1.0% of the iodobutane contains radioactive iodine. The specific rotation of this recovered iodobutane is found to be +15.58°.
b. What does this result suggest about the mechanism of the reaction of 2-iodobutane with iodide ion?
Problem 49a
Optically active 2-bromobutane undergoes racemization on treatment with a solution of KBr. Propose a mechanism for this racemization.
Problem 49a,b
a. Optically active 2-bromobutane undergoes racemization on treatment with a solution of KBr. Give a mechanism for this racemization.
b. In contrast, optically active butan-2-ol does not racemize on treatment with a solution of KOH. Explain why a reaction like that in part (a) does not occur.
Problem 49b
In contrast, optically active butan-2-ol does not racemize on treatment with a solution of KOH. Explain why a reaction like that in part (a) does not occur.
Problem 49c
Optically active butan-2-ol racemizes in dilute acid. Propose a mechanism for this racemization.
Problem 50
Give a mechanism to explain the two products formed in the following reaction.
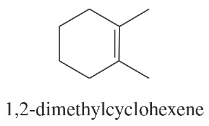
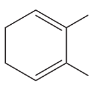
Problem 50f
Using 1,2-dimethylcyclohexene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.) If a chiral product is shown, assume that it is part of a racemic mixture.
(f)
Problem 52
Because the SN1 reaction goes through a flat carbocation, we might expect an optically active starting material to give a completely racemized product. In most cases, however, SN1 reactions actually give more of the inversion product. In general, as the stability of the carbocation increases, the excess inversion product decreases. Extremely stable carbocations give completely racemic products. Explain these observations.
Problem 53
Triethyloxonium tetrafluoroborate, (CH3CH2)3O+ BF4–, is a solid with melting point 91–92°C. Show how this reagent can transfer an ethyl group to a nucleophile (Nuc:−) in an SN2 reaction. What is the leaving group? Why might this reagent be preferred to using an ethyl halide? (Consult Table 6-2)
Problem 54
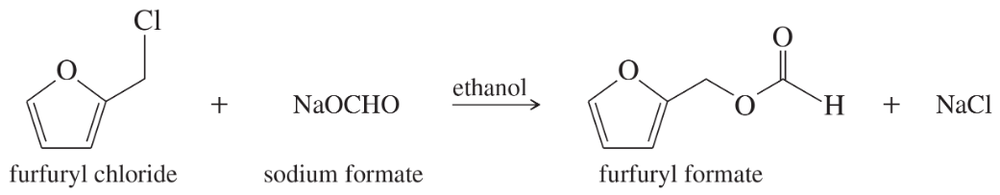
Furfuryl chloride can undergo substitution by both SN2 and SN1 mechanisms. Because it is a 1° alkyl halide, we expect SN2 but not SN1 reactions. Draw a mechanism for the SN1 reaction shown below, paying careful attention to the structure of the intermediate. How can this primary halide undergo SN1 reactions?
Problem 56
The following reaction takes place under second-order conditions (strong nucleophile), yet the structure of the product shows rearrangement. Also, the rate of this reaction is several thousand times faster than the rate of substitution of hydroxide ion on 2-chlorobutane under similar conditions. Propose a mechanism to explain the enhanced rate and rearrangement observed in this unusual reaction. (“Et” is the abbreviation for ethyl.)
Problem 57a
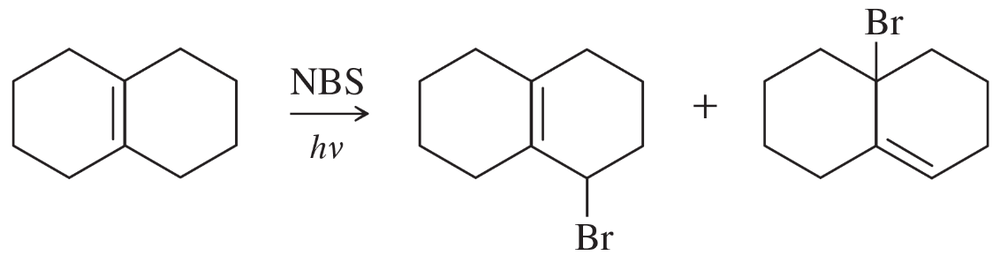
Propose mechanisms to account for the observed products in the following reactions.
(a)
Problem 57b
Propose mechanisms to account for the observed products in the following reactions.
(b)
Problem 58a,b
Show the products you expect when each compound reacts with NBS with light shining on the reaction.
(a)
(b)
Problem 58c
Show the products you expect when each compound reacts with NBS with light shining on the reaction.
(c)
Problem 59
A student adds NBS to a solution of 1-methylcyclohexene and irradiates the mixture with a sunlamp until all the NBS has reacted. After a careful distillation, the product mixture contains two major products of formula C7H11Br.
(a) Draw the resonance forms of the three possible allylic free radical intermediates.
(b) Rank these three intermediates from most stable to least stable.
(c) Draw the products obtained from each free-radical intermediate.
Problem 59a
A student adds NBS to a solution of 1-methylcyclohexene and irradiates the mixture with a sunlamp until all the NBS has reacted. After a careful distillation, the product mixture contains two major products of formula C7H11Br.
a. Draw the resonance forms of the three possible allylic free radical intermediates.
Problem 59b
A student adds NBS to a solution of 1-methylcyclohexene and irradiates the mixture with a sunlamp until all the NBS has reacted. After a careful distillation, the product mixture contains two major products of formula C7H11Br.
b. Rank these three intermediates from most stable to least stable.