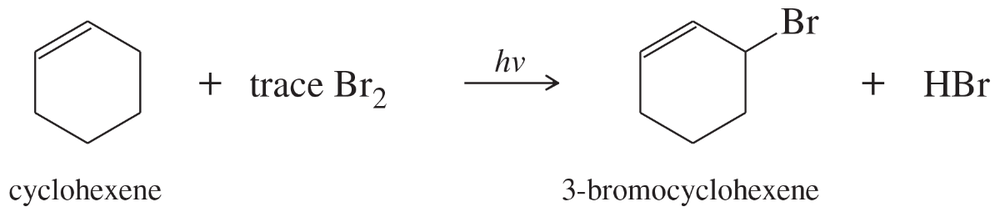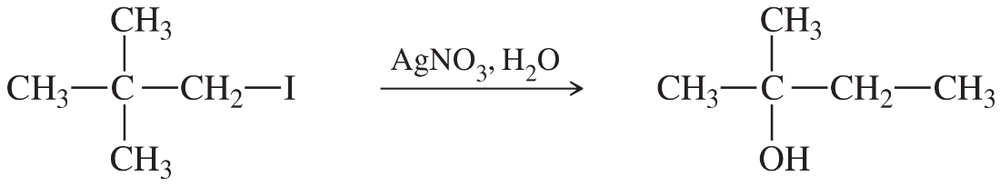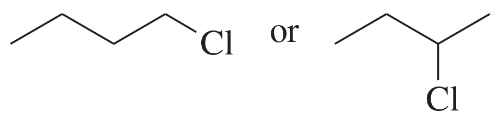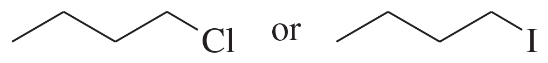Back
BackProblem 25d
In the presence of a small amount of bromine, cyclohexene undergoes the following light-promoted reaction:
d. Explain why cyclohexene reacts with bromine much faster than cyclohexane, which must be heated to react.
Problem 26b
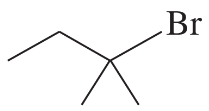
Propose a mechanism involving a hydride shift or an alkyl shift for each solvolysis reaction. Explain how each rearrangement forms a more stable intermediate.
Hint: Most rearrangements convert 2° (or incipient 1°) carbocations to 3° or resonance-stabilized carbocations.
(b)
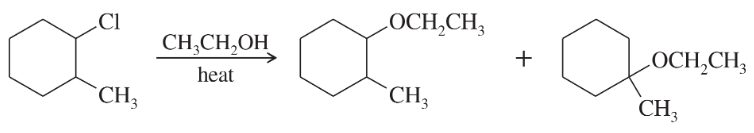
Problem 26c
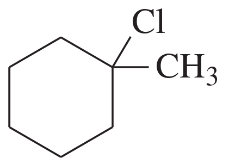
Propose a mechanism involving a hydride shift or an alkyl shift for each solvolysis reaction. Explain how each rearrangement forms a more stable intermediate.
Hint: Most rearrangements convert 2° (or incipient 1°) carbocations to 3° or resonance-stabilized carbocations.
(c)
Problem 26d
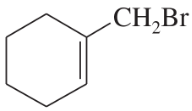
Propose a mechanism involving a hydride shift or an alkyl shift for each solvolysis reaction. Explain how each rearrangement forms a more stable intermediate.
Hint: Most rearrangements convert 2° (or incipient 1°) carbocations to 3° or resonance-stabilized carbocations.
(d)
Problem 27a,b
For each reaction, give the expected substitution product, and predict whether the mechanism will be predominantly first order (SN1) or second order (SN2).
a. 2-chloro-2-methylbutane + CH3COOH
b. isobutylbromide + sodium methoxide
Problem 27c
For each reaction, give the expected substitution product, and predict whether the mechanism will be predominantly first order (SN1) or second order (SN2).
c. 1-iodo-1-methylcyclohexane + ethanol
Problem 27d,e
For each reaction, give the expected substitution product, and predict whether the mechanism will be predominantly first order (SN1) or second order (SN2).
d. cyclohexylbromide + methanol
e. cyclohexylbromide + sodium ethoxide
Problem 28
Under certain conditions, when (R)-2-bromobutane is heated with water, the SN1 substitution proceeds twice as fast as the SN2. Calculate the e.e. and the specific rotation expected for the product. The specific rotation of (R)-butan-2-ol is −13.5°. Assume that the SN1 gives equal amounts of the two enantiomers.
Problem 29a
A reluctant first-order substrate can be forced to ionize by adding some silver nitrate (one of the few soluble silver salts) to the reaction. Silver ion reacts with the halogen to form a silver halide (a highly exothermic reaction), generating the cation of the alkyl group.
Give mechanisms for the following silver-promoted rearrangements.
(a)
Problem 29b
A reluctant first-order substrate can be forced to ionize by adding some silver nitrate (one of the few soluble silver salts) to the reaction. Silver ion reacts with the halogen to form a silver halide (a highly exothermic reaction), generating the cation of the alkyl group.
Give mechanisms for the following silver-promoted rearrangements.
(b)
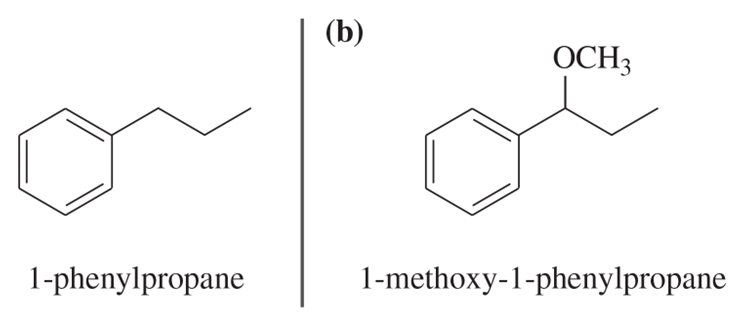
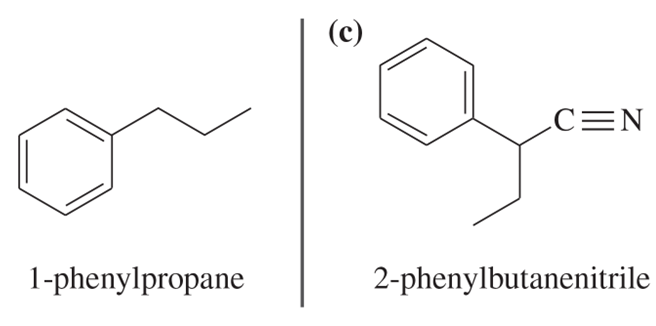
Problem 30b
Show how you would convert (in one or two steps) 1-phenylpropane to the three products shown below. In each case, explain what unwanted reactions might produce undesirable impurities in the product.
Problem 30c
Show how you would convert (in one or two steps) 1-phenylpropane to the three products shown below. In each case, explain what unwanted reactions might produce undesirable impurities in the product.
Problem 31a,b
Draw the structures of the following compounds.
a. sec-butyl chloride
b. isobutyl bromide
Problem 33a,b
Predict the compound in each pair that will undergo the SN2 reaction faster.
(a)
(b)
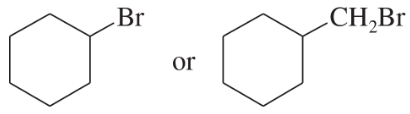
Problem 33c,d
Predict the compound in each pair that will undergo the SN2 reaction faster.
(c)
(d)
Problem 34a,b
Predict the compound in each pair that will undergo solvolysis (in aqueous ethanol) more rapidly.
(a) (CH3CH2)2CH—Cl or (CH3)3C—Cl
(b)
Problem 34c,d
Predict the compound in each pair that will undergo solvolysis (in aqueous ethanol) more rapidly.
(c)
(d)
Problem 35e,f
Show how each compound might be synthesized by the SN2 displacement of an alkyl halide.
e. H2C=CH—CH2CN
f. H—C≡C—CH2CH2CH3
Problem 36
Give two syntheses for (CH3)2CH—O—CH2CH3, and explain which synthesis is better.
Problem 37a
When ethyl bromide is added to potassium tert-butoxide, the product is ethyl tert-butyl ether.
CH3CH2–Br + (CH3)3C–O–K+ → (CH3)3C–O–CH2CH3 ethyl bromide potassium tert-butoxide ethyl tert-butyl ether
a. What happens to the reaction rate if the concentration of ethyl bromide is doubled?
Problem 37b,c
When ethyl bromide is added to potassium tert-butoxide, the product is ethyl tert-butyl ether.
CH3CH2–Br + (CH3)3C–O–K+ → (CH3)3C–O–CH2CH3 ethyl bromide potassium tert-butoxide ethyl tert-butyl ether
b. What happens to the rate if the concentration of potassium tert-butoxide is tripled and the concentration of ethyl bromide is doubled?
c. What happens to the rate if the temperature is raised?
Problem 38a
When tert-butyl bromide is heated with an equal amount of ethanol in an inert solvent, one of the products is ethyl tert-butyl ether.
a. What happens to the reaction rate if the concentration of ethanol is doubled?
Problem 38b,c
When tert-butyl bromide is heated with an equal amount of ethanol in an inert solvent, one of the products is ethyl tert-butyl ether.
b. What happens to the rate if the concentration of tert-butyl bromide is tripled and the concentration of ethanol is doubled?
c. What happens to the rate if the temperature is raised?
Problem 39
Chlorocyclohexane reacts with sodium cyanide (NaCN) in ethanol to give cyanocyclohexane. The rate of formation of cyanocyclohexane increases when a small amount of sodium iodide is added to the solution. Explain this acceleration in the rate.
Problem 40a,b
Give the substitution products expected from solvolysis of each compound by heating in ethanol.
(a)
(b)
Problem 40c,d
Give the substitution products expected from solvolysis of each compound by heating in ethanol.
(c)
(d)
Problem 41a
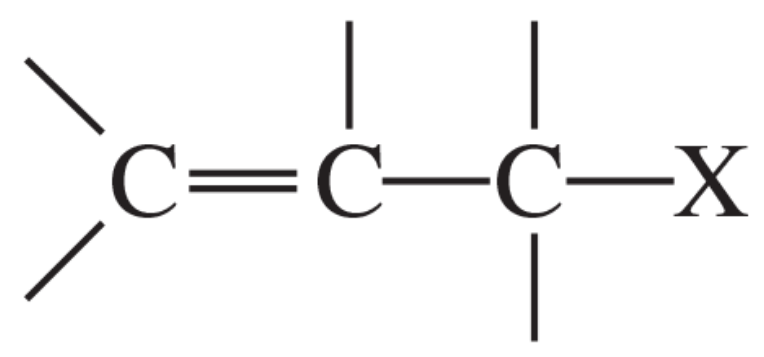
Allylic halides have the structure
a. Show how the first-order ionization of an allylic halide leads to a resonance-stabilized cation.
Problem 41b(i,ii)
Allylic halides have the structure
b. Draw the resonance structures of the allylic cations formed by ionization of the following halides.
(i)
(ii)
Problem 41c
Allylic halides have the structure
c. Show the products expected from SN1 solvolysis of these halides in ethanol.
Problem 42
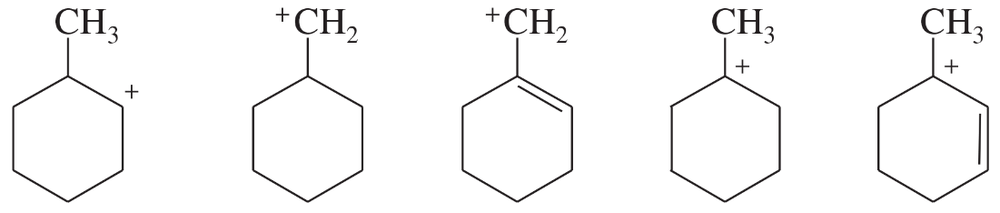
List the following carbocations in decreasing order of their stability.