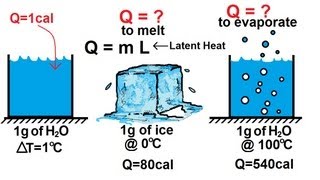
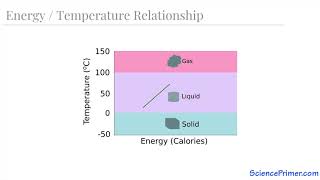
20. Heat and Temperature
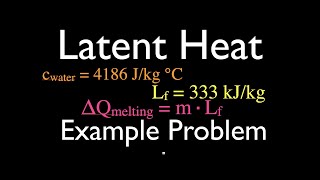
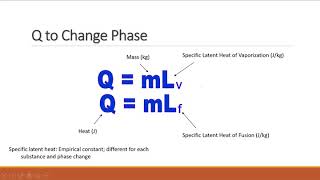
Latent Heat & Phase Changes
Learn with other creators
Practice this topic
- Multiple Choice
How much heat must be removed from 0.7 kg of water at 23°C to cool it to 0°C and completely freeze it?
800views16rank1comments - Multiple ChoiceHow much heat is needed to change of liquid water at into steam at ?585views
- Textbook Question
A 5.0 g ice cube at −20°C is in a rigid, sealed container from which all the air has been evacuated. How much heat is required to change this ice cube into steam at 200°C? Steam has cV = 1500 J/kg K and cP = 1960 J/kg K.
857views - Textbook Question
High-altitude mountain climbers do not eat snow, but always melt it first with a stove. To see why, calculate the energy absorbed from your body if you melt 1.0 kg of -15°C snow using a stove and drink the resulting 1.0 kg of water at 2°C, which your body has to warm to 37°C.
293views - Textbook Question
An iron boiler of mass 180 kg contains 710 kg of water at 18°C. A heater supplies energy at the rate of 58,000 kJ/h. How long does it take for the water to all have changed to steam?
385views - Textbook Question
Calculate what will happen when 1000 J of heat is added to 100 grams of water at 10°C.
375views - Multiple Choice
Why does the evaporation of sweat cool your body on a warm day?
358views