21. Kinetic Theory of Ideal Gases
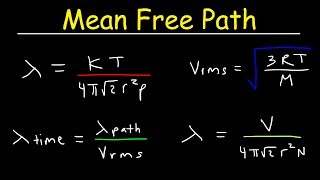
Mean Free Path of Gases
Learn with other creators
Practice this topic
- Multiple Choice
Laboratory environments can achieve pressures of 3.5×10-13 atm and temperatures of 300K. Calculate the mean free path (in km) of air molecules, which you can assume are diatomic.
695views3rank - Textbook Question
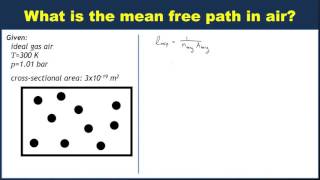
Calculate the mean free path of air molecules at atm and K. (This pressure is readily attainable in the laboratory; see Exercise .) As in Example , model the air molecules as spheres of radius m.
2113views - Textbook Question
On earth, STP is based on the average atmospheric pressure at the surface and on a phase change of water that occurs at an easily produced temperature, being only slightly cooler than the average air temperature. The atmosphere of Venus is almost entirely carbon dioxide (CO2), the pressure at the surface is a staggering 93 atm, and the average temperature is 470℃. Venusian scientists, if they existed, would certainly use the surface pressure as part of their definition of STP. To complete the definition, they would seek a phase change that occurs near the average temperature. Conveniently, the melting point of the element tellurium is 450℃. What are (a) the rms speed and (b) the mean free path of carbon dioxide molecules at Venusian STP based on this phase change in tellurium? The radius of a CO2 molecule is 1.5 x 10-10 m.
763views - Textbook Question
Photons of light scatter off molecules, and the distance you can see through a gas is proportional to the mean free path of photons through the gas. Photons are not gas molecules, so the mean free path of a photon is not given by Equation 20.3, but its dependence on the number density of the gas and on the molecular radius is the same. Suppose you are in a smoggy city and can barely see buildings 500 m away. How far would you be able to see if all the molecules around you suddenly doubled in volume?
781views - Textbook Question
A mad engineer builds a cube, 2.5 m on a side, in which 6.2-cm-diameter rubber balls are constantly sent flying in random directions by vibrating walls. He will award a prize to anyone who can figure out how many balls are in the cube without entering it or taking out any of the balls. You decide to shoot 6.2-cm-diameter plastic balls into the cube, through a small hole, to see how far they get before colliding with a rubber ball. After many shots, you find they travel an average distance of 1.8 m. How many rubber balls do you think are in the cube?
696views - Multiple Choice
In the context of the mean free path of gases, which expression best represents the mean free time between collisions, , for electrons moving in an aluminum wire, given the mean free path and the average speed of the electrons?
59views - Multiple Choice
Given that the mean free path of a helium atom in helium gas at standard temperature and pressure is , which of the following changes would most likely decrease the mean free path of the helium atoms?
68views