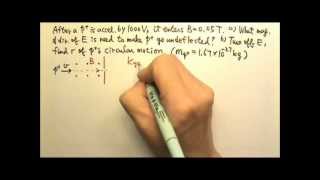28. Magnetic Fields and Forces
Mass Spectrometer
Learn with other creators
Practice this topic
- Multiple Choice
A negative charge in a spectrometer is accelerated in the negative x-axis. It is later deflected and collides some distance ABOVE velocity selector. What are the orientations of the electric and magnetic fields, respectively, inside the selector?
1328views16rank3comments - Multiple Choice
A 2 kg, −3 C charge is accelerated through a potential difference of 4 V. The velocity selector has an electric field of magnitude 5 N/C. How far from the velocity selector will the charge collide against the spectrometer "wall"?
1336views17rank2comments - Textbook Question
(II) Protons would move in a circle of radius 5.10 cm perpendicular to a 0.585-T magnetic field. What value of electric field could make their paths straight? In what direction must the electric field point?
871views - Textbook Question
(II) Suppose the electric field between the electric plates in the mass spectrometer of Fig. 27–34 is 2.84 x 104 V/m and the magnetic fields are B = B' = 0.58 T. The source contains carbon isotopes of mass numbers 12, 13, and 14 from a long-dead piece of a tree. (To estimate atomic masses, multiply by 1.67 x 10-27 kg.) How far apart are the marks formed by the singly charged ions of each type on a detector or photographic film?
960views - Textbook Question
Suppose the electric field between the electric plates in the mass spectrometer of Fig. 27–34 is 2.84 x 10⁴ V/m and the magnetic fields are B = B'= 0.58 T. The source contains carbon isotopes of mass numbers 12, 13, and 14 from a long-dead piece of a tree. (To estimate atomic masses, multiply by 1.67 x 10⁻²⁷ kg .) How far apart are the marks formed by the singly charged ions of each type on a detector or photographic film? What if the ions were doubly charged?
736views - Textbook Question
Suppose the electric field between the electric plates in the mass spectrometer of Fig. 27–34 is 2.84 x 10⁴ V/m and the magnetic fields are B = B'= 0.58 T. The source contains carbon isotopes of mass numbers 12, 13, and 14 from a long-dead piece of a tree. (To estimate atomic masses, multiply by 1.67 x 10⁻²⁷ kg.) Does it matter if the ion charge is positive (lost electrons) or negative (gained electrons)? Explain.
1015views - Multiple Choice
In a mass spectrometer, which of the following particles is considered to have negligible mass?
461views