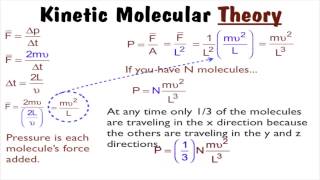
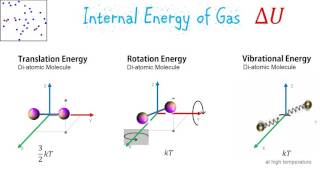
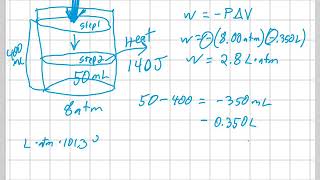21. Kinetic Theory of Ideal Gases
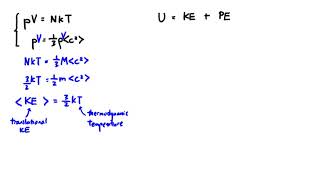
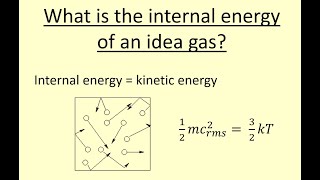
Internal Energy of Gases
Learn with other creators
Practice this topic
- Multiple Choice
A container filled with 2 mol of an ideal, monoatomic gas is has a total internal energy equal to the kinetic energy of a 0.008kg bullet travelling at 700 m/s. What is the temperature of the gas in Kelvin?
971views5rank - Textbook Question
Compute the specific heat at constant volume of nitrogen (N2) gas, and compare it with the specific heat of liquid water. The molar mass of N2 is g/mol.
1604views - Textbook Question
How much heat does it take to increase the temperature of mol of an ideal gas by K near room temperature if the gas is held at constant volume and is monatomic?
717views - Textbook Question
How much heat does it take to increase the temperature of mol of an ideal gas by K near room temperature if the gas is held at constant volume and is diatomic?
1086views - Textbook Question
A 100 cm³ box contains helium at a pressure of 2.0 atm and a temperature of 100℃. It is placed in thermal contact with a 200 cm³ box containing argon at a pressure of 4.0 atm and a temperature of 400℃. What is the final thermal energy of each gas?
995views