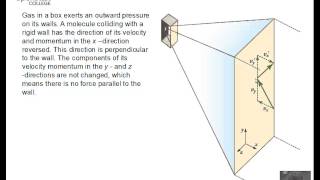
21. Kinetic Theory of Ideal Gases
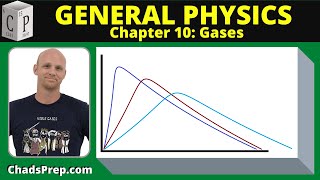
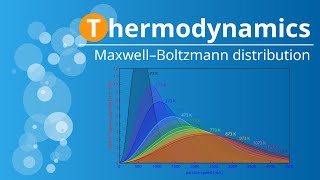
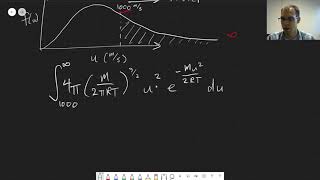
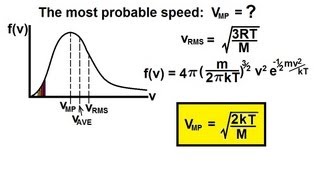
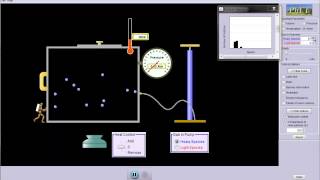
Speed Distribution of Ideal Gases
Learn with other creators
Practice this topic
- Multiple Choice
The escape velocity from the Earth is approximately 11.2 km/s. If the mass of helium atoms is 6.64 × 10-27 kg, at what temperature would the average speed of helium atoms be equal to the escape velocity?
1159views2rank1comments - Textbook Question
For a gas of nitrogen molecules (N2), what must the temperature be if of all the molecules have speeds less than m/s? Use Table . The molar mass of N2 is g/mol.
1233views - Textbook Question
For diatomic carbon dioxide gas (CO2, molar mass g/mol) at K, calculate the average speed .
968views - Textbook Question
For diatomic carbon dioxide gas (CO2, molar mass g/mol) at K, calculate the most probable speed .
1290views1rank - Textbook Question
(II) A group of 25 particles have the following speeds: two have speed 10 m/s, seven have 15 m/s, four have 20 m/s, three have 25 m/s, six have 30 m/s, one has 35 m/s, and two have 40 m/s. Determine the average speed.
519views