Back
BackProblem 1
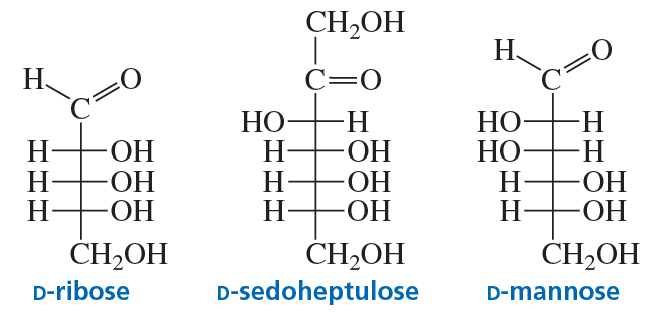
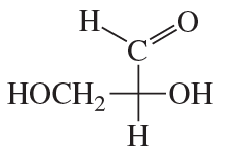
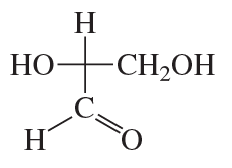
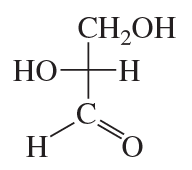
Classify the following monosaccharides:
Problem 2
Draw Fischer projections of L-glucose and L-fructose.
Problem 3
Indicate whether each of the following structures is D-glyceraldehyde or L-glyceraldehyde, assuming that the horizontal bonds point toward you and the vertical bonds point away from you (Section 4.7):
a.
b.
c.
Problem 4
a. Are d-erythrose and l-erythrose enantiomers or diastereomers?
b. Are l-erythrose and l-threose enantiomers or diastereomers?
Problem 5c
c. What sugar is the C-4 epimer of L-gulose?
Problem 5d
d. What sugar is the C-4 epimer of D-lyxose?
Problem 6a
What are the systematic names of the following compounds? Indicate the configuration (R or S) of each asymmetric center.
a. D-glucose
Problem 6b
What are the systematic names of the following compounds? Indicate the configuration (R or S) of each asymmetric center.
b. D-mannose
Problem 7
What sugar is the C-3 epimer of D-fructose?
Problem 8a
How many stereoisomers are possible for a. a ketoheptose?
Problem 8b
How many stereoisomers are possible for b. an aldoheptose?
Problem 11
When D-tagatose is added to a basic aqueous solution, an equilibrium mixture of monosaccharides is obtained, two of which are aldohexoses and two of which are ketohexoses. Identify the aldohexoses and ketohexoses.
Problem 13b
What monosaccharide is reduced to two alditols, one of which is the alditol obtained from the reduction of
1. D-talose?
2. D-allose?
Problem 14a,b
a. Name an aldohexose other than D-glucose that is oxidized to D-glucaric acid by nitric acid.
b. What is another name for D-glucaric acid?
Problem 14c
Name another pair of aldohexoses that are oxidized to identical aldaric acids.
Problem 15a
What monosaccharides are formed in a modified Kiliani–Fischer synthesis starting with each of the following monosaccharides?
a. D-xylose
Problem 16a
What two monosaccharides can be degraded to
a. D-ribose?
Problem 16b
What two monosaccharides can be degraded to
b. D-arabinose?
Problem 19b
4-Hydroxy- and 5-hydroxyaldehydes exist primarily as cyclic hemiacetals. Draw the structure of the cyclic hemiacetal formed by each of the following:
b. 4-hydroxypentanal
Problem 19c
4-Hydroxy- and 5-hydroxyaldehydes exist primarily as cyclic hemiacetals. Draw the structure of the cyclic hemiacetal formed by each of the following:
c. 5-hydroxypentanal