Back
BackProblem 43
Propose a mechanism for the formation of d-allose from d-glucose in a basic solution.
Problem 44
Treatment with sodium borohydride converts aldose A to an optically inactive alditol. Wohl degradation of A forms B, whose alditol is optically inactive. Wohl degradation of B forms D-glyceraldehyde. Identify A and B.
Problem 45
The disaccharide lactulose consists of a D-galactopyranose subunit and a D-fructofuranose subunit joined by a β-1,4′-glycosidic linkage. After treatment of lactulose with 1. excess CH3I/Ag2O, 2. HCl/H2O, the d-galactopyranose subunit was found to have one nonmethylated OH group, whereas the D-fructofuranose subunit had two. Draw the structure of ⍺-lactulose.
Problem 46
A hexose was obtained after (+)-glyceraldehyde underwent three successive Kiliani–Fischer syntheses. Identify the hexose from the following experimental information: oxidation with nitric acid forms an optically active aldaric acid; a Wohl degradation followed by oxidation with nitric acid forms an optically inactive aldaric acid; and a second Wohl degradation forms erythrose.
Problem 47
Draw the mechanism for the interconversion of α-D-glucose and β-D-glucose in dilute HCl.
Problem 53
A D-aldopentose is oxidized by nitric acid to an optically active aldaric acid. A Wohl degradation of the aldopentose leads to a monosaccharide that is oxidized by nitric acid to an optically inactive aldaric acid. Identify the D-aldopentose.
Problem 54
Draw the mechanism for the formation of β-lactose from ⍺-D-galactose and β-D-glucose in dilute HCl.
Problem 55
Hyaluronic acid, a component of connective tissue, is the fluid that lubricates joints. It is a polymer of alternating N-acetyl-D-glucosamine and D-glucuronic acid subunits joined by β-1,3′-glycosidic linkages. Draw a short segment of hyaluronic acid.
Problem 59
The aldaric acid of D-glucose forms two five-membered-ring lactones. Draw their structures.
Problem 61
How many aldaric acids are obtained from the 16 aldohexoses?
Problem 62
A hexose is obtained when the residue of a shrub Sterculia setigeria undergoes acid-catalyzed hydrolysis. Identify the hexose from the following experimental information: it undergoes mutarotation; it does not react with Br2; and D-galactonic acid and D-talonic acid are formed when it reacts with Tollens’ reagent.
Problem 64a,b
Draw each of the following:
a. β-D-talopyranose
b. α-D-idopyranose
Problem 65
Calculate the percentages of -D-glucose and -D-glucose present at equilibrium from the specific rotations of -D-glucose, -D-glucose, and the equilibrium mixture. Compare your values with those given in Section 20.10. (Hint: The specific rotation of the mixture equals the specific rotation of -D-glucose times the fraction of glucose present in the a-form plus the specific rotation of -D-glucose times the fraction of glucose present in the -form.)
Problem 66
The specific rotation of α-D-galactose is 150.7 and that of β-D-galactose is 52.8. When an aqueous mixture that was initially 70% α-D-galactose and 30% β-D-galactose reaches equilibrium, the specific rotation is 80.2. What is the percentage of α-D-galactose and β-D galactose at equilibrium?
Problem 67
Draw the mechanism for the elimination step in the Wohl degredation.
Problem 68
An unknown disaccharide gives a positive Tollens' test. A glycosidase hydrolyzes it to D-galactose and D-mannose. When the disaccharide is treated with methyl iodide and Ag2O and then hydrolyzed with dilute HCl, the products are 2,3,4,6-tetra-O-methylgalactose and 2,3,4-tri-O-methylmannose. Propose a structure for the disaccharide.
Problem 69
Predict whether D-altrose exists preferentially as a pyranose or a furanose. (Hint: In the most stable arrangement for a five-membered ring, all the adjacent substituents are trans.)
Problem 70
Trehalose, C12H22O11, is a nonreducing sugar that is only 45% as sweet as sugar. When hydrolyzed by aqueous acid or the enzyme maltase, it forms only D-glucose. When it is treated with excess methyl iodide in the presence of Ag2O and then hydrolyzed with water under acidic conditions, only 2,3,4,6-tetra-O-methyl-d-glucose is formed. Draw the structure of trehalose.
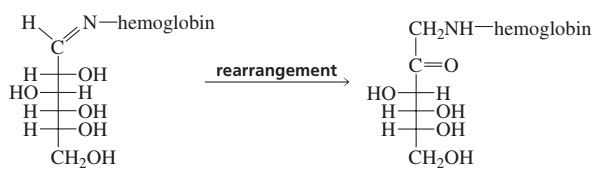
Problem 71
Propose a mechanism for the rearrangement that converts an ⍺-hydroxyimine to an ⍺-aminoketone in the presence of a trace amount of acid.
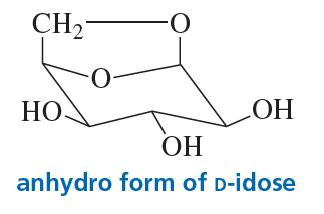
Problem 73
When a pyranose is in the chair conformation in which the CH2OH group and the C-1 OH group are both in axial positions, the two groups can react to form an acetal. This is called the anhydro form of the sugar (it has 'lost water'). The anhydro form of d-idose is shown here. Explain why about 80% of d-idose exists in the anhydro form in an aqueous solution at 100 °C, but only about 0.1% of d-glucose exists in the anhydro form under the same conditions.