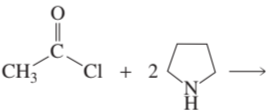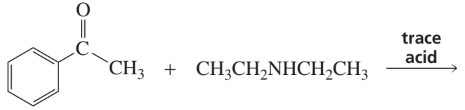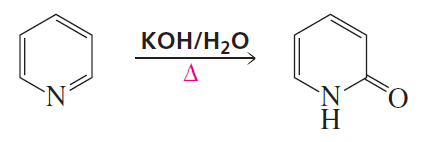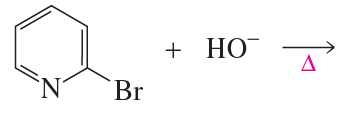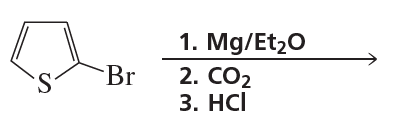Back
BackProblem 1c,d
Name the following:
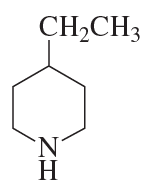
c.
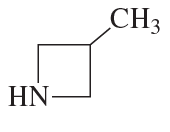
d.
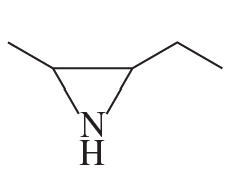
Problem 1e
Name the following:
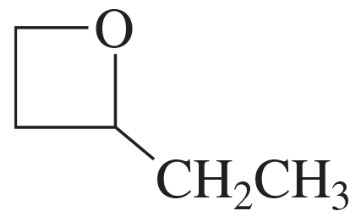
e.
Problem 1f
Name the following:
f.
Problem 3
Why is the conjugate acid of morpholine more acidic than the conjugate acid of piperidine?
Problem 5b
Draw the product of each of the following reactions:
b.
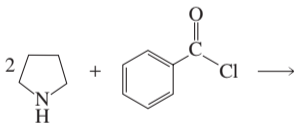
Problem 5d
Draw the product of each of the following reactions:
d.
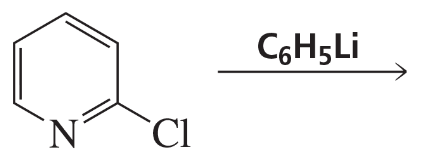
Problem 7
Explain why cyclopentadiene (pKa = 15) is more acidic than pyrrole (pKa ∼17), even though nitrogen is more electronegative than carbon.
Problem 8
When pyrrole is added to a dilute solution of D2SO4 in D2O, 2-deuteriopyrrole is formed. Propose a mechanism to account for the formation of this compound.
Problem 10a
Draw the product formed when pyridine reacts with ethyl bromide
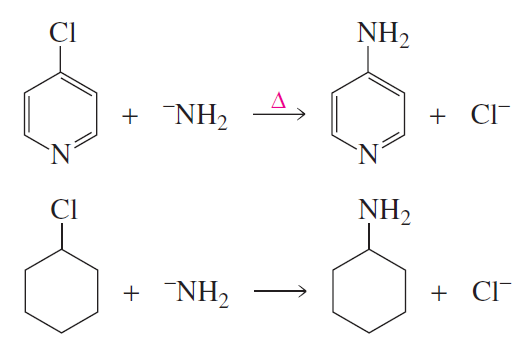
Problem 11
How do the mechanisms of the following reactions differ?
Problem 12a
Propose a mechanism for the following reaction:
Problem 12b
What other product is formed in this reaction?
Problem 13
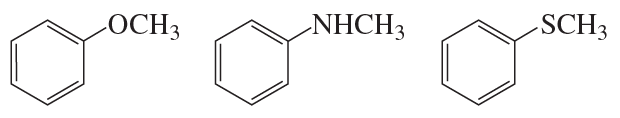
Rank the following compounds from easiest to hardest at removing a proton from its methyl substituent:
Problem 15
Rank imidazole, pyrrole, and benzene from most reactive to least reactive toward electrophilic aromatic substitution.
Problem 16a
Imidazole boils at 257 °C, whereas N-methylimidazole boils at 199 °C. Explain the difference in boiling points.
Problem 17
Why is imidazole a stronger acid (pKa ~ 14.4) than pyrrole (pKa ~ 17)?
Problem 18a
What percent of imidazole is protonated at physiological pH (7.4)?
Problem 20
Why is protonated pyrimidine (pKa = 1.0) more acidic than protonated pyridine (pKa = 5.2)?
Problem 22a
What are the products of the following reactions?
a.
Problem 22b
What are the products of the following reactions?
b.
Problem 22d
What are the products of the following reactions?
d.
Problem 22e
What are the products of the following reactions?
e.
Problem 22g
What are the products of the following reactions?
g.
Problem 22i
What are the products of the following reactions?
i.
Problem 25
Rank the following compounds from most reactive to least reactive in an electrophilic aromatic substitution reaction:
Problem 26
One of the following compounds undergoes electrophilic aromatic substitution predominantly at C-3, and one undergoes electrophilic aromatic substitution predominantly at C-4. Which is which?
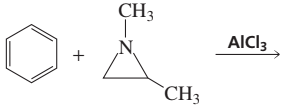
Problem 27
Benzene undergoes electrophilic aromatic substitution reactions with aziridines in the presence of a Lewis acid such as AlCl3.
a. What are the major and minor products of the following reaction?
b. Would you expect epoxides to undergo similar reactions?
Problem 28
Pyrrole reacts with excess para-(N,N-dimethylamino)benzaldehyde to form a highly colored compound. Draw the structure of the colored compound.
Problem 30a,b
Name the following:
a.
b.
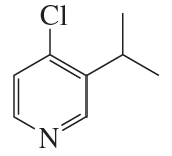
Problem 30c
Name the following:
c.