Back
BackProblem 19d
4-Hydroxy- and 5-hydroxyaldehydes exist primarily as cyclic hemiacetals. Draw the structure of the cyclic hemiacetal formed by each of the following:
d. 4-hydroxyheptanal
Problem 21
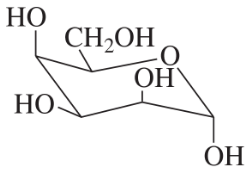
D-Glucose most often exists as a pyranose, but it can also exist as a furanose. Draw the Haworth projection of α-D-glucofuranose
Problem 22
Draw the anomers of D-erythrofuranose.
Problem 22-23
When glucose undergoes base-catalyzed isomerization in the absence of the enzyme, mannose is one
of the products that is formed (Section 20.5). Why is mannose not formed in the enzyme-catalyzed reaction?
Problem 24
Which OH groups are in the axial position in each of the following?
a. β-D-idopyranose
b. α-D-allopyranose
Problem 25
Draw the products formed when β-D-galactose reacts with ethanol and HCl. (Show all structures as chair conformers.)
Problem 26
Why is only a trace amount of acid used in the formation of an N-glycoside?
Problem 28a
Name the following compounds and indicate whether or not each is a reducing sugar:
a.
Problem 28b
Name the following compounds and indicate whether or not each is a reducing sugar:
b.
Problem 28c
Name the following compounds and indicate whether or not each is a reducing sugar:
c.
Problem 29
What is the specific rotation of an equilibrium mixture of fructose? (Hint: Recall that the specific rotation of an equilibrium mixture of glucose is +52.7.)
Problem 30a
a. Identify the sugars in amygdalin.
Problem 30b
b. Identify the glycosidic linkage that connects the sugars.
Problem 31a
What is the main structural difference between a. amylose and cellulose?
Problem 31b
What is the main structural difference between b. amylose and amylopectin?
Problem 31c
What is the main structural difference between c. amylopectin and glycogen?
Problem 31d
What is the main structural difference between d. cellulose and chitin?
Problem 32
Explain why the C-3 OH group of vitamin C is more acidic than the C-2 OH group.
Problem 33a
Refer to Figure 20.5 to answer the following questions: a. People with type O blood can donate blood to anyone, but they cannot receive blood from everyone. From whom can they not receive blood?
Problem 33b
Refer to Figure 20.5 to answer the following questions: b. People with type AB blood can receive blood from anyone, but they cannot give blood to everyone. To whom can they not give blood?
Problem 34b
What product or products are obtained when D-galactose reacts with each of the following?
b. Ag+, NH3, HO-
Problem 34c
What product or products are obtained when D-galactose reacts with each of the following?
c. NaBH4, followed by H3O+
Problem 34d
What product or products are obtained when d-galactose reacts with each of the following?
d. excess CH3I + Ag2O
Problem 34e
What product or products are obtained when D-galactose reacts with each of the following?
e. Br2 in water
Problem 34f
What product or products are obtained when D-galactose reacts with each of the following?
f. ethanol + HCl
Problem 34g
What product or products are obtained when d-galactose reacts with each of the following?
g.
1. hydroxylamine/trace acid
2. acetic anhydride/∆
3. HO-/H2O
Problem 35
Name the epimers of D-glucose.
Problem 36a
Identify the sugar in each description.
a. An aldopentose that is not D-arabinose forms D-arabinitol when it is reduced with NaBH4.
Problem 36b
Identify the sugar in each description.
b. A sugar that is not D-altrose forms D-altraric acid when it is oxidized with nitric acid.
Problem 36c
Identify the sugar in each description.
c. A ketose that, when reduced with NaBH4, forms D-altritol and D-allitol.