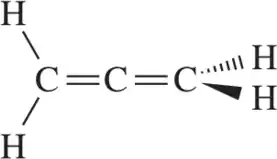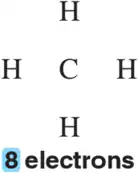Back
BackProblem 40d
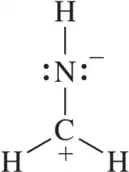
Use hybrid orbitals to draw the following molecules.
(d)
Problem 41
Rank the following molecules by the length of the indicated bond from shortest to longest.
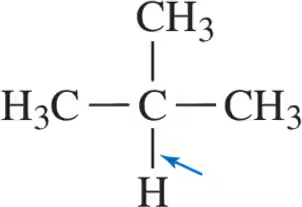
(a)
(b)
(c)
Problem 43
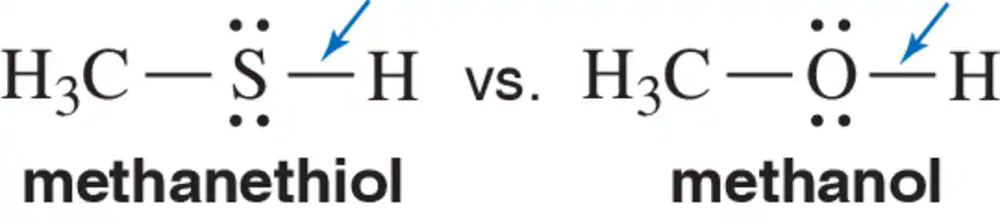
The sulfur and oxygen in methanethiol and methanol are both sp3 hybridized. Why is the S―H bond longer than the O―H bond?
Problem 44
A molecular orbital diagram is shown for the C―Cl bond in chloromethane. If two more electrons were added to chloromethane, where would the electrons go?
<IMAGE>
Problem 45
How might electrons be excited from π to π* based on the molecular orbital diagram shown? [This will be relevant in Chapter 21.]
<IMAGE>
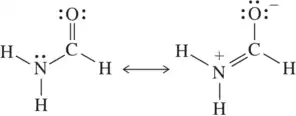
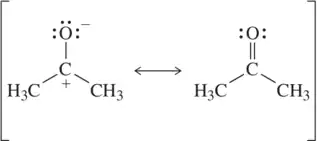
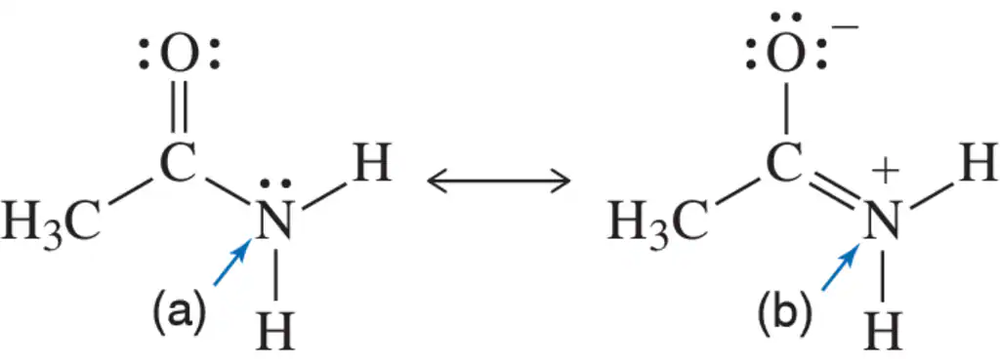
Problem 47a
Each pair of structures represents two valid resonance structures. Use the arrow-pushing formalism to justify the formation of the one on the left from the one on the right.
(a)
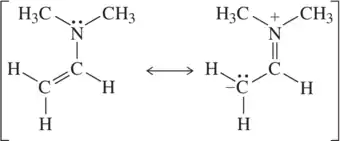
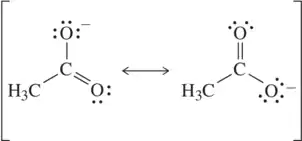
Problem 47b
Each pair of structures represents two valid resonance structures. Use the arrow-pushing formalism to justify the formation of the one on the left from the one on the right.
(b)
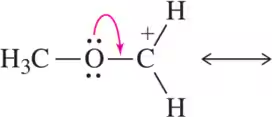
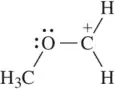
Problem 48a
Draw the resonance structure that would result from the indicated movement of electrons.
(a)
Problem 49a
For each of the molecules shown, do the following:
(i) Identify all pushable pairs.
(ii) Identify all places where electrons can be pushed.
(iii) Draw one valid resonance structure.
(a)
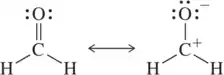
Problem 50
i) Which of the following resonance structures represents the 'actual' structure of the molecule shown? (ii) Which contributes more to the resonance hybrid? (iii) Why?
(a)
(b)
(c)
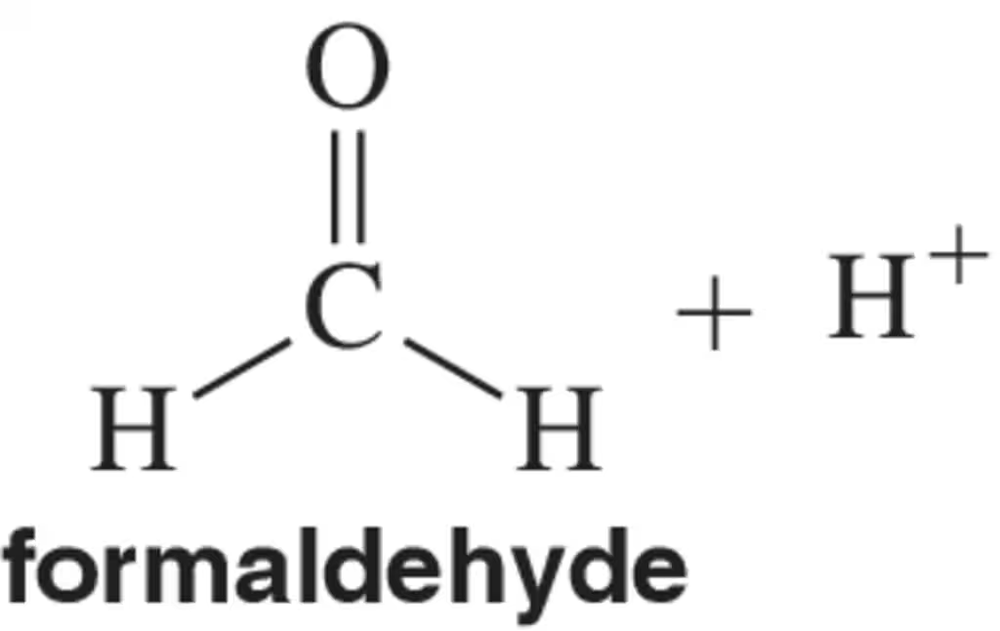
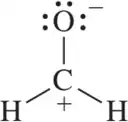
Problem 51
To which atom of formaldehyde would you expect H+ to add?
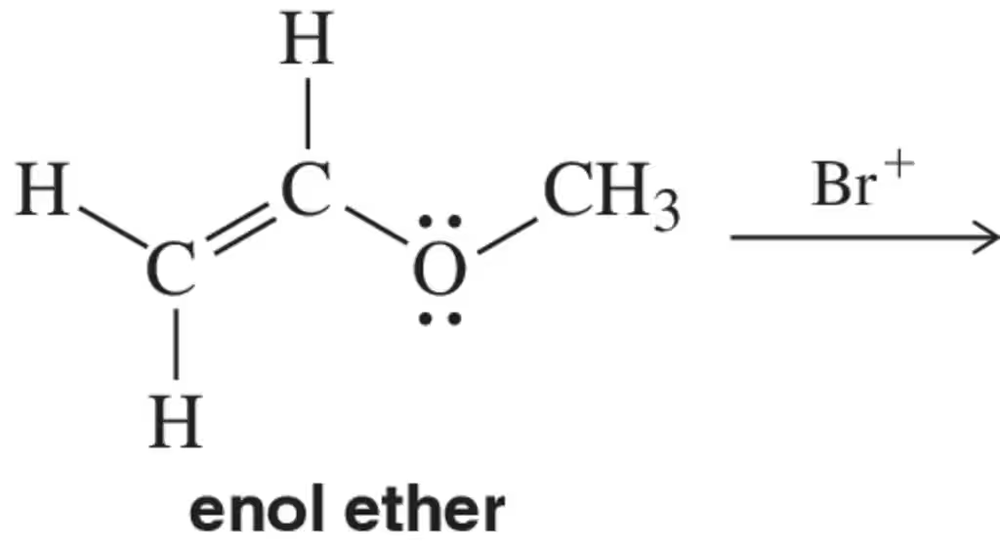
Problem 52
Which atoms in the enol ether would you expect to react with Br⁺ ?
Problem 53
Assign the hybridization of the nitrogen in each resonance structure of acetamide.
Problem 55c
How many valence electrons does each of the following contribute to a Lewis structure?
(c) B
Problem 55g
How many valence electrons does each of the following contribute to a Lewis structure?
(g) S
Problem 55i
How many valence electrons does each of the following contribute to a Lewis structure?
(i) Cl
Problem 55j
How many valence electrons does each of the following contribute to a Lewis structure?
(j) Al
Problem 55l
How many valence electrons does each of the following contribute to a Lewis structure?
(l) ―1 charge
Problem 58b
Show how the Lewis dot structure for each of the following atoms would overlap to form a single bond.
(b) C and O
Problem 59b
Which atom in each pair would you expect to be the central atom in a Lewis structure?
(b) C vs. O
Problem 59d
Which atom in each pair would you expect to be the central atom in a Lewis structure?
(d) C vs. N
Problem 59f
Which atom in each pair would you expect to be the central atom in a Lewis structure?
(f) C vs. F
Problem 60a
Given the atoms involved and the number of valence electrons remaining, complete the Lewis structure by placing bonds between atoms such that each has a full octet.
(a)
Problem 60d
Given the atoms involved and the number of valence electrons remaining, complete the Lewis structure by placing bonds between atoms such that each has a full octet.
(d)
Problem 60e
Given the atoms involved and the number of valence electrons remaining, complete the Lewis structure by placing bonds between atoms such that each has a full octet.
(e)
Problem 61a
By moving an electron pair, draw a better Lewis structure that minimizes formal charges.
(a)
Problem 61b
By moving an electron pair, draw a better Lewis structure that minimizes formal charges.
(b)
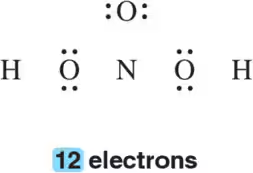
Problem 62j
Draw the Lewis structure for the following molecular formulas.
(j) H2SO4
Problem 62l
Draw the Lewis structure for the following molecular formulas.
(l) SO42-
Problem 62u
Draw the Lewis structure for the following molecular formulas.
(u) NH4+