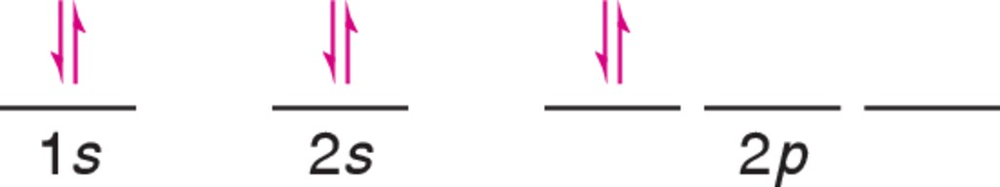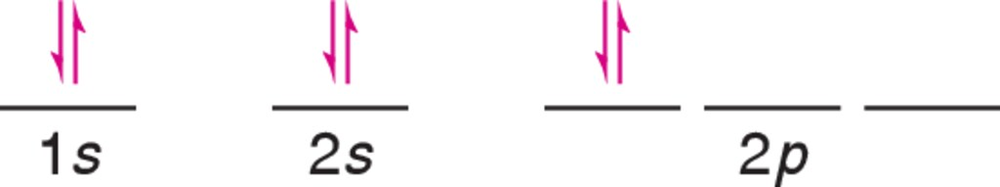Back
BackProblem 1b
Give the electron configuration of the following elements.
(b) N
Problem 1c
Give the electron configuration of the following elements.
(c) O
Problem 1d
Give the electron configuration of the following elements.
(d) F
Problem 2a
Why do elements in the same group of the periodic table display similar reactivity?
Problem 3a
Rank the following elements from least electronegative to most electronegative.
Na, Si, F, Mg, C, O, N
Problem 4a
How many valence shell electrons do each of the following elements contain? How many new bonds can each form?
(a) C
Problem 4b
How many valence shell electrons do each of the following elements contain? How many new bonds can each form?
(b) N
Problem 4c
How many valence shell electrons do each of the following elements contain? How many new bonds can each form?
(c) O
Problem 5
Why is argon considered to be so stable that it is referred to as a noble gas?
Problem 7
Bromine-79 (50.7% abundance) has an atomic mass of 78.918 amu, whereas bromine-81 (49.3% abundance) has an atomic mass of 80.916 amu. From these data, calculate the average atomic mass of bromine that you would expect to see in the periodic table.
Problem 8
Chlorine exists as one of two isotopes with atomic masses of 34.969 amu (35Cl) and 36.966 amu (37Cl) . Calculate the relative abundance of 35Cl and 37Cl based on the average atomic mass of 35.453 amu.
Problem 10a
A chemistry student drew the following incorrect electron configuration for carbon. (a) Correct the diagram.
Problem 10b
A chemistry student drew the following incorrect electron configuration for carbon. (b) Which rule wasn't followed by the student?
Problem 12
Would you expect electrons in the 2s or 3s orbital to be more reactive? Why?
Problem 13b
Choose the larger atom in each pair.
(b) O or F
Problem 13d
Choose the larger atom in each pair.
(d) N or S
Problem 14a
How many electrons does an atom of each of the following elements need to lose to achieve a noble gas configuration? By losing that many electrons, which noble gas configuration is achieved?
(a) Beryllium
Problem 14b
How many electrons does an atom of each of the following elements need to lose to achieve a noble gas configuration? By losing that many electrons, which noble gas configuration is achieved?
(b) Aluminum
Problem 14c
How many electrons does an atom of each of the following elements need to lose to achieve a noble gas configuration? By losing that many electrons, which noble gas configuration is achieved?
(c) Magnesium
Problem 14d
How many electrons does an atom of each of the following elements need to lose to achieve a noble gas configuration? By losing that many electrons, which noble gas configuration is achieved?
(d) Potassium
Problem 15a
How many electrons does an atom of each of the following elements need to gain to achieve a noble gas configuration? By gaining that many electrons, which noble gas configuration is achieved?
(a) Oxygen
Problem 15b
How many electrons does an atom of each of the following elements need to gain to achieve a noble gas configuration? By gaining that many electrons, which noble gas configuration is achieved?
(b) Iodine
Problem 15c
How many electrons does an atom of each of the following elements need to gain to achieve a noble gas configuration? By gaining that many electrons, which noble gas configuration is achieved?
(c) Phosphorus
Problem 15d
How many electrons does an atom of each of the following elements need to gain to achieve a noble gas configuration? By gaining that many electrons, which noble gas configuration is achieved?
(d) Chlorine
Problem 16a
Give the charge most likely to result from ionization of the following metals.
(a) Lithium
Problem 16b
Give the charge most likely to result from ionization of the following metals.
(b) Aluminum
Problem 16c
Give the charge most likely to result from ionization of the following metals.
(c) Potassium
Problem 17b
Give the charge most likely to result from ionization of the following nonmetals.
(b) Sulfur
Problem 18a
Show the ionic compound that you would expect to form between the given metal and nonmetal. Label the charges on each species.
(a) Al and F
Problem 18b
Show the ionic compound that you would expect to form between the given metal and nonmetal. Label the charges on each species.
(b) Mg and Br