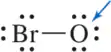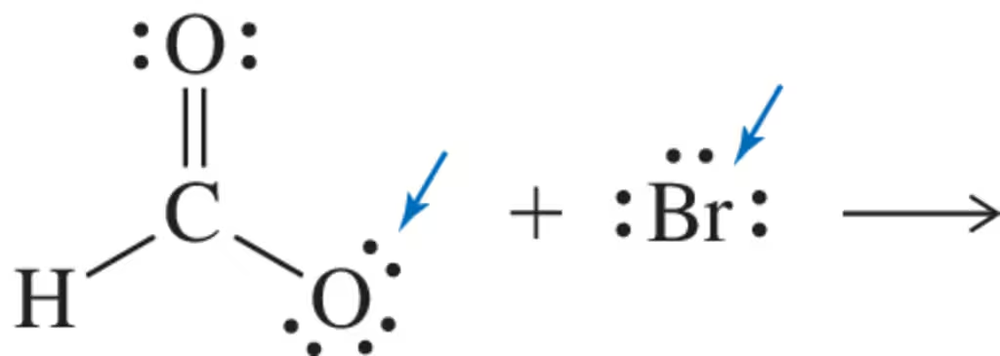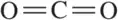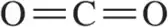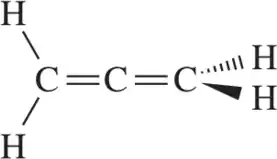Back
BackProblem 19
Fill in the blank. Oxygen ionizes to become ― 2 instead of ― 1 because O2- is more ___________ .
Problem 20b
(i) Using the periodic trend, choose the more electronegative atom in each pair. For one pair, you'll need to look at the actual Pauling values. (ii) For which one? (iii) Why?
(b) N vs. O
Problem 20d
(i) Using the periodic trend, choose the more electronegative atom in each pair. For one pair, you'll need to look at the actual Pauling values. (ii) For which one? (iii) Why?
(d) B vs. Si
Problem 23
Based on your answer to Assessment 2.22, would you expect a larger atom to be more or less electronegative than a smaller atom?
Problem 24a
Without looking at Figure 2.20, use your intuition to estimate whether a bond is ionic, polar covalent, or covalent.
(a) Na―Cl
Problem 25
Using only your intuition, rank the following covalent bonds in terms of their polarity (1 = most polar; 6 = least polar). [You can use the periodic trend of electronegativity, but don't use actual numbers.]
(a) C―C
(b) C―O
(c) C―H
(d) C―F
(e) C―Cl
(f) C―S
Problem 27b
Draw the Lewis structure for the following molecules. Be sure to calculate the formal charge of each atom as a way of confirming your structure is correct.
(b) HOBr
Problem 27c
Draw the Lewis structure for the following molecules. Be sure to calculate the formal charge of each atom as a way of confirming your structure is correct.
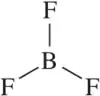
(c) CF4
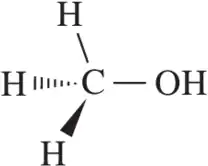
Problem 28d
Calculate the formal charge of the indicated atom in the following molecules or ions.
(d)
Problem 28e
Calculate the formal charge of the indicated atom in the following molecules or ions.
(e)
Problem 28f
Calculate the formal charge of the indicated atom in the following molecules or ions.
(f)
Problem 29b
Draw the Lewis structure for the following ions. Be sure to calculate the formal charge of each atom to confirm that your structure is correct.
(b) H+
Problem 29c
Draw the Lewis structure for the following ions. Be sure to calculate the formal charge of each atom to confirm that your structure is correct.
(c) BH4-
Problem 29d
Draw the Lewis structure for the following ions. Be sure to calculate the formal charge of each atom to confirm that your structure is correct.
(d) AlCl4-
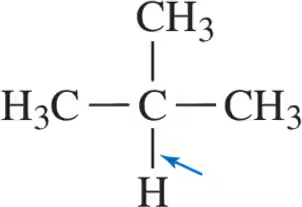
Problem 30a
Calculate the formal charge of the indicated atoms in the ions shown.
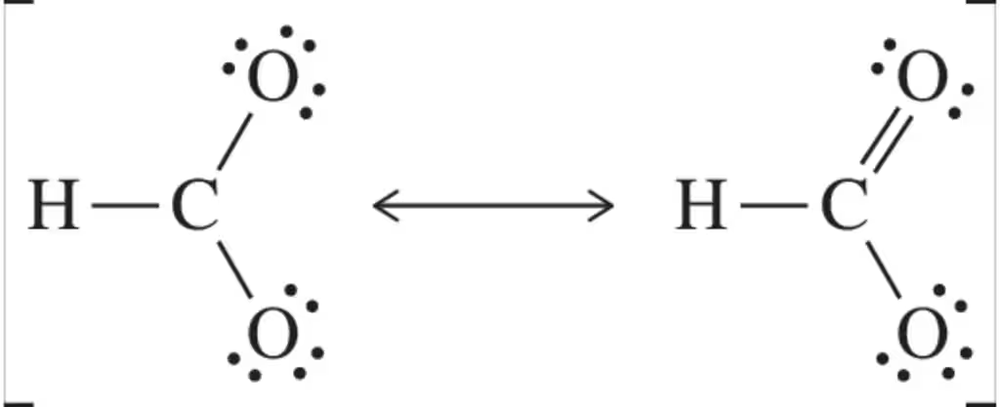
Problem 33
Calculate the formal charge on the non-hydrogen atoms in the molecules shown. Use the arrow-pushing formalism to 'move' an electron pair such that it is shared between two (formerly) charged atoms. Your arrow should account for the formation of the molecule on the right from the molecule on the left.
Problem 35c
You drew the Lewis structures of the following compounds and ion in Assessment 2.32. Predict their shapes around the central atom based on the Lewis structure.
(c) HCO2H
Problem 35d
You drew the Lewis structures of the following compounds and ion in Assessment 2.32. Predict their shapes around the central atom based on the Lewis structure.
(d) CO32-
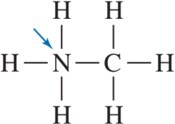
Problem 36a
For the molecules shown, indicate the direction of the dipole moment.
(a)
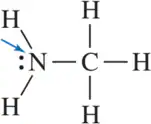
Problem 36b
For the molecules shown, indicate the direction of the dipole moment.
(b)
Problem 37
Hydrogen forms bonds without hybridizing. Why?
Problem 40a
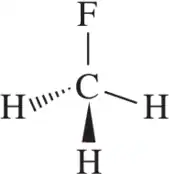
Use hybrid orbitals to draw the following molecules.
(a)
Problem 40b
Use hybrid orbitals to draw the following molecules.
(b)
Problem 40c
Use hybrid orbitals to draw the following molecules.
(c)
Problem 40d
Use hybrid orbitals to draw the following molecules.
(d)
Problem 41
Rank the following molecules by the length of the indicated bond from shortest to longest.
(a)
(b)
(c)
Problem 43
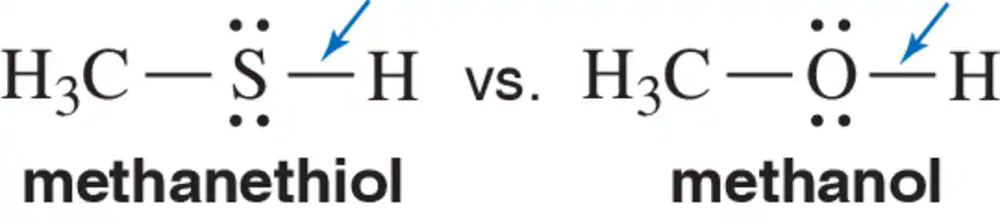
The sulfur and oxygen in methanethiol and methanol are both sp3 hybridized. Why is the S―H bond longer than the O―H bond?
Problem 44
A molecular orbital diagram is shown for the C―Cl bond in chloromethane. If two more electrons were added to chloromethane, where would the electrons go?
<IMAGE>
Problem 45
How might electrons be excited from π to π* based on the molecular orbital diagram shown? [This will be relevant in Chapter 21.]
<IMAGE>
Problem 47a
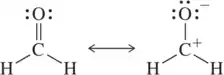
Each pair of structures represents two valid resonance structures. Use the arrow-pushing formalism to justify the formation of the one on the left from the one on the right.
(a)