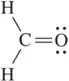
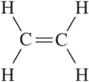
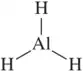
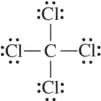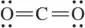Back
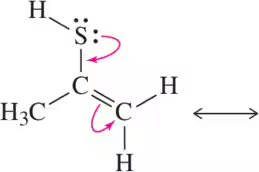
BackProblem 67a
Identify the resonance structure that will be produced given the molecule shown and the electron flow indicated.
(a)
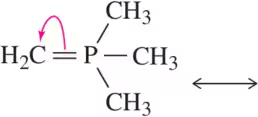
Problem 67c
Identify the resonance structure that will be produced given the molecule shown and the electron flow indicated.
(c)
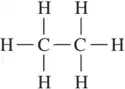
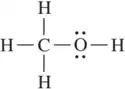
Problem 70a,b
Refer to the following Lewis structures.
(a)
(b)
Predict the hybridization of all non-hydrogen atoms.
Problem 70c,d
Predict the hybridization of all non-hydrogen atoms.
(c)
(d)
Problem 70e,f,g
Predict the hybridization of all non-hydrogen atoms.
(e)
(f)
(g)
Problem 72a,b
Refer to the following Lewis structures.
(a)
(b)
If an atom is sp² hybridized, how many sp²-hybridized orbitals does it use for bonding? How many p orbitals?
Problem 72c,d
Refer to the following Lewis structures.
(c)
(d)
If an atom is sp² hybridized, how many sp²-hybridized orbitals does it use for bonding? How many p orbitals?
Problem 74b
Connect the atoms using the indicated hybrid orbitals.
(b) C (sp2) with C (sp2)
Problem 75c
For the following partial structures, the bond is shown. Add the indicated number of bonds, being sure to specify the orientation (that is, x, y, or z axis) of the p orbitals used.
(c)
Problem 75e
For the following partial structures, the σ bond is shown. Add the indicated number of π bonds, being sure to specify the orientation (that is, x, y, or z axis) of the p orbitals used.
(e)
Problem 77a
Given the Lewis structures, indicate the direction of the dipole moment, if there is one.
(a)
Problem 77b
Given the Lewis structures, indicate the direction of the dipole moment, if there is one.
(b)
Problem 77e
Given the Lewis structures, indicate the direction of the dipole moment, if there is one.
(e)
Problem 78
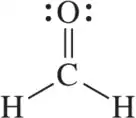
Carbon dioxide (CO2) has no dipole moment (μ = 0 D). The related molecule sulfur dioxide (SO2) does have a dipole moment (μ = 1.6 D) Explain this observation.
Problem 79
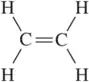
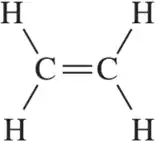
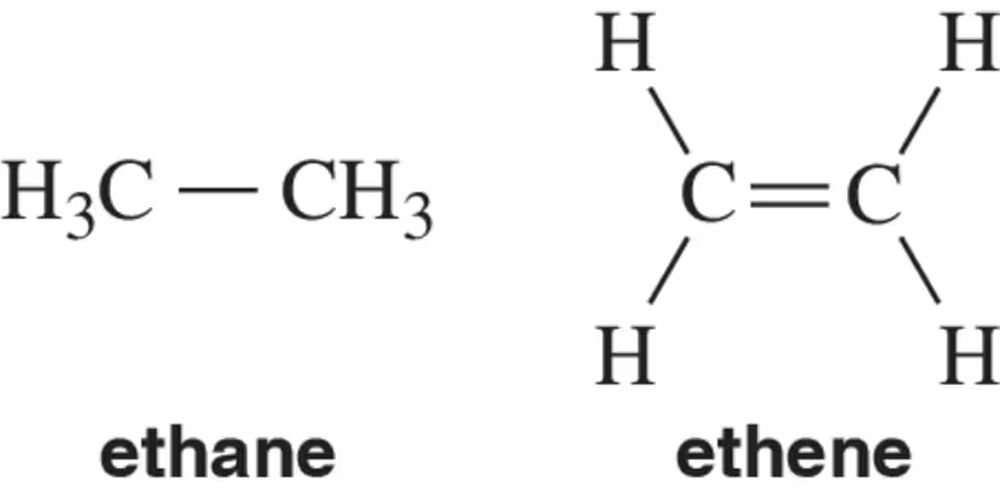
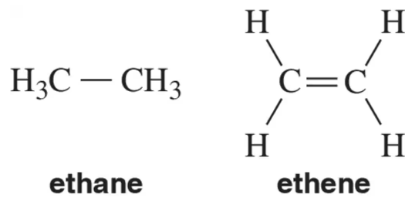
There is free rotation around the C―C bond in ethane. There is an extremely high barrier to rotation around the C=C bond in in ethene. Explain.
Problem 80
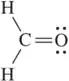
The C―H σ bond length in ethane is 1.09 Å. The C―H σ bond in ethene is 1.07 Å. Explain.
Problem 81
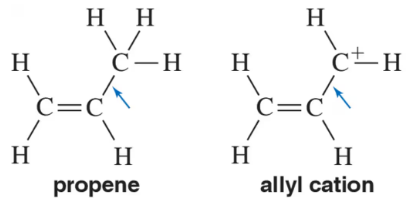
In propene, the indicated C―C bond length is 1.51 Å. In the allyl cation, the indicated C―C bond length is 1.41 Å. Explain.
Problem 82
Looking ahead in Chapter 4, we explain that molecules like CH3+ are Lewis acids or electron pair acceptors. Into which orbital would the new electron pair go?
Problem 83
In comparison to CH3+ in Assessment 2.82, the related molecule H3O+ is not a Lewis acid at oxygen. Why?
Problem 84
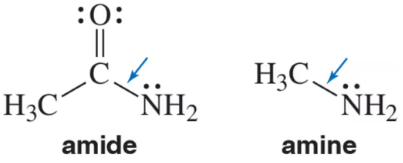
The C―N bond in the following amide is much stronger than the C―N bond in the amine. Explain.
Problem 85a
Looking ahead in Chapter 3, we describe how the formal charge on an atom can be used to predict the number of lone pairs. Given the charge, or lack of charge, on each atom, fill in the electron pairs.
(a)
Problem 85c
Looking ahead in Chapter 3, we describe how the formal charge on an atom can be used to predict the number of lone pairs. Given the charge, or lack of charge, on each atom, fill in the electron pairs.
(c)
Problem 86
In Chapter 4, we explain that molecules like CH3- are Lewis bases or electron pair donors. What makes it a Lewis base?
Problem 87
In comparison to CH3- in Assessment 2.86, the related molecule BH4- is itself not a Lewis base at boron. Why?