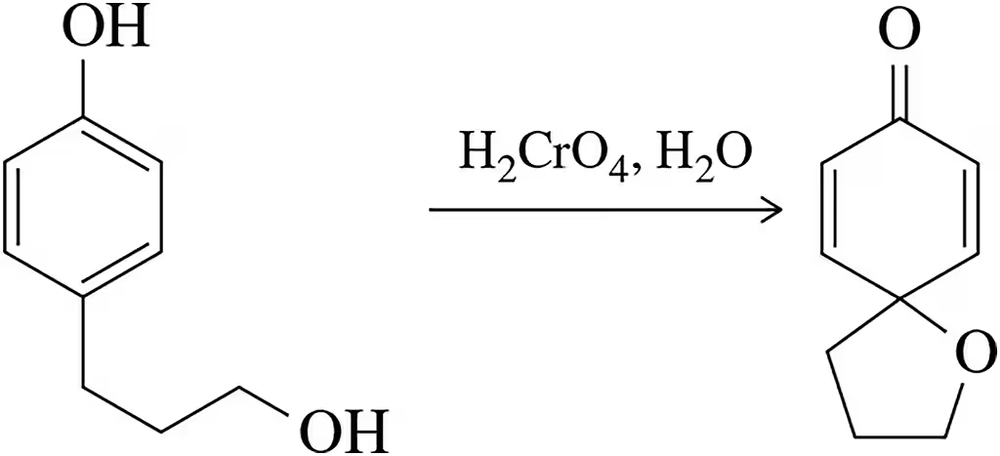Back
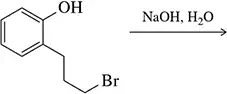
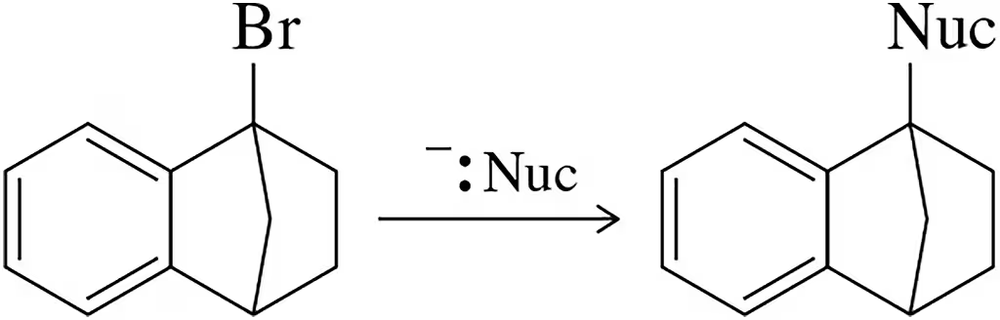
BackProblem 33a
Predict the product of the following reactions.
(a)
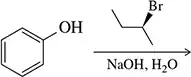
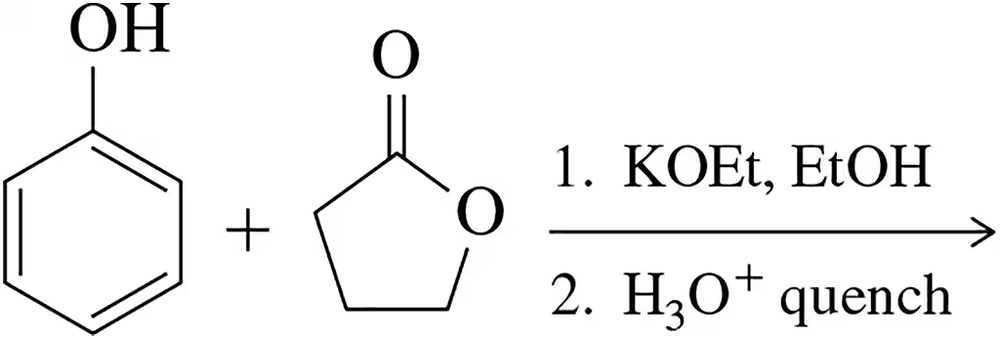
Problem 33b
Predict the product of the following reactions.
(b)
Problem 34b
Predict the product(s) of the following reactions.
(b)
Problem 34d
Predict the product(s) of the following reactions.
(d)
Problem 34f
Predict the product(s) of the following reactions.
(f)
Problem 34g
Predict the product(s) of the following reactions.
(g)
Problem 34h
Predict the product(s) of the following reactions.
(h)
Problem 34j
Predict the product(s) of the following reactions.
(j)
Problem 34k
Predict the product(s) of the following reactions.
(k)
Problem 35a
Suggest the reagents used to effect the transformations shown.
(a)
Problem 35c
Suggest the reagents used to effect the transformations shown.
(c)
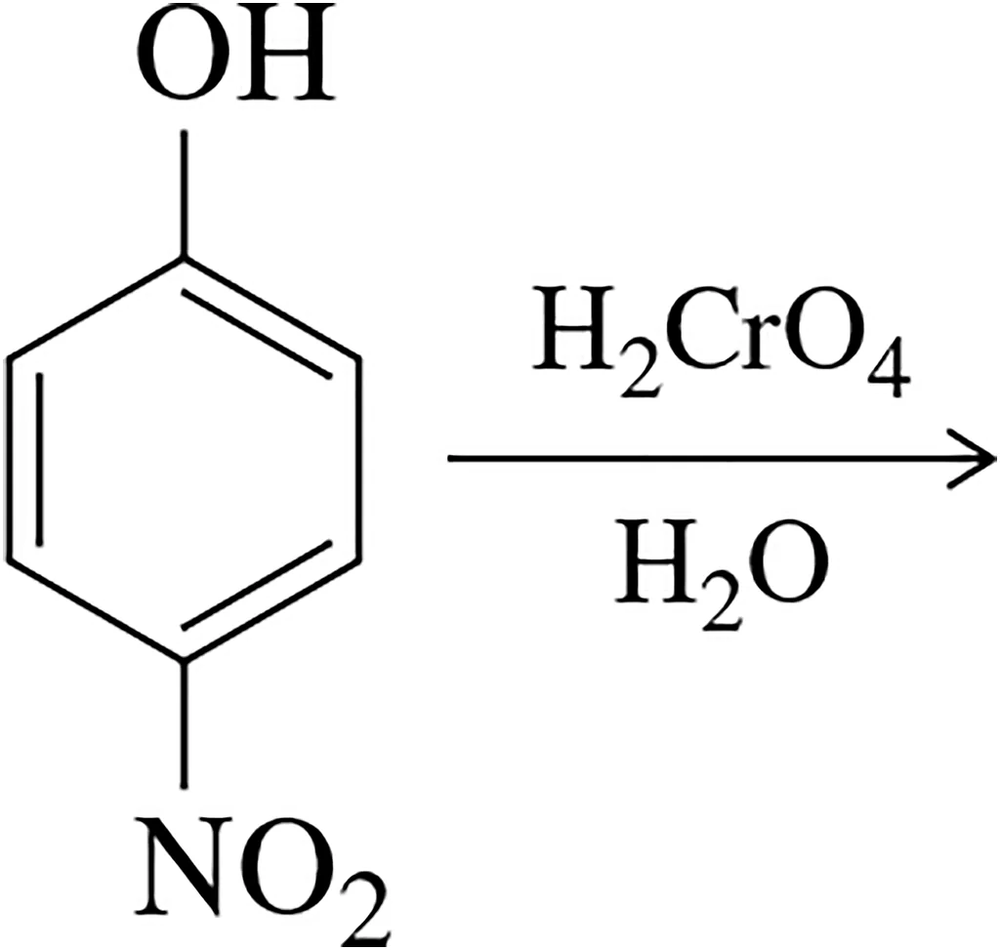
Problem 37
Oxidation of the phenol shown gives a single quinone product. Predict this product and explain why it is the only one formed.
Problem 38
In the second propagation step in the bromination of toluene, Br2 is only attacked by a radical on the substituent carbon. Why?
Problem 39
When (R)-(1-bromoethyl)benzene is treated with sodium cyanide, a single enantiomer is produced. However, upon treatment of the same molecule with water, a mixture of two enantiomers is obtained.
(a) Explain these results.
(b) Why is only partial racemization sometimes observed?
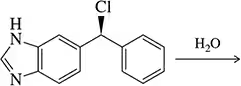
Problem 40
The benzylic bromide shown undergoes neither SN1 nor SN2 substitution reactions. Explain.
Problem 41
In Chapter 13, we learned that epoxide opening can give different products, depending on whether the reaction occurs under acidic or basic conditions. Explain why the epoxide shown opens identically under either set of conditions.
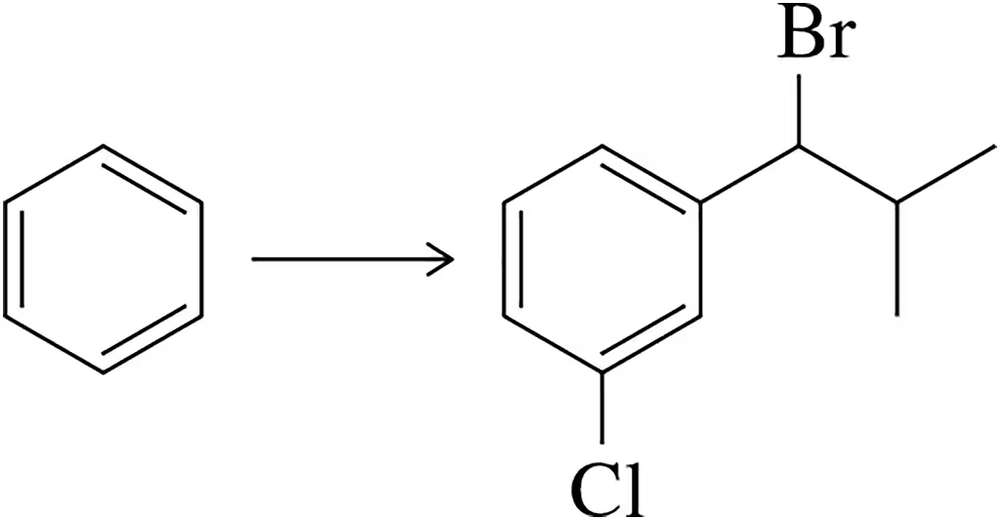
Problem 43
Beginning with benzene, synthesize the benzyl bromide shown.
Problem 46
The following reaction proceeds in good yield, despite requiring high temperature and high concentration. Identify the product you’d expect to obtain in this reaction.
Problem 47
The following steps were used in the synthesis of the antimalarial thiaplakortone A. Identify the missing reagents, (a) and (b),
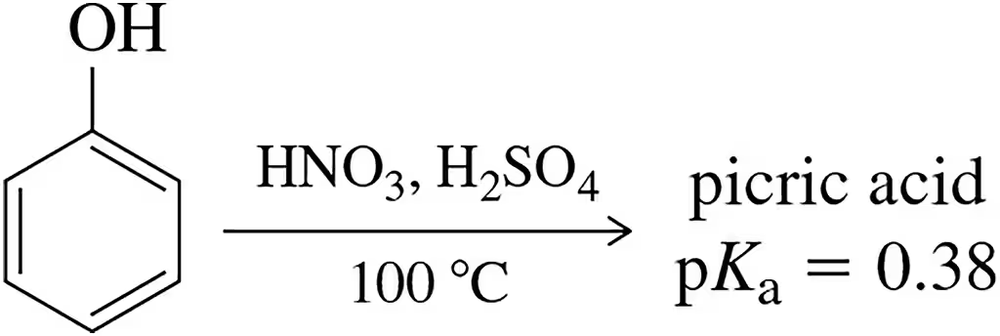
Problem 50
In Chapter 23, we learned about the electrophilic aromatic nitration reaction. When phenol is subjected to these conditions with a large excess of nitric acid, a molecule called picric acid is produced. Predict the product of this reaction and explain why the pKa value of this compound is 0.38.
Problem 51
Rationalize the fact that 1,4-dihydroxybenzene melts at a significantly higher temperature than 1,2-diydroxybenzene.
Problem 52
A chemist attempted to oxidize a primary alcohol to a carboxylic acid using chromic acid. The product shown was obtained as a major component in the mixture. Suggest an arrow-pushing mechanism that accounts for its formation. [Think about what chromic acid would normally do to a phenol and make a list of bonds formed and bonds broken.]
Problem 53
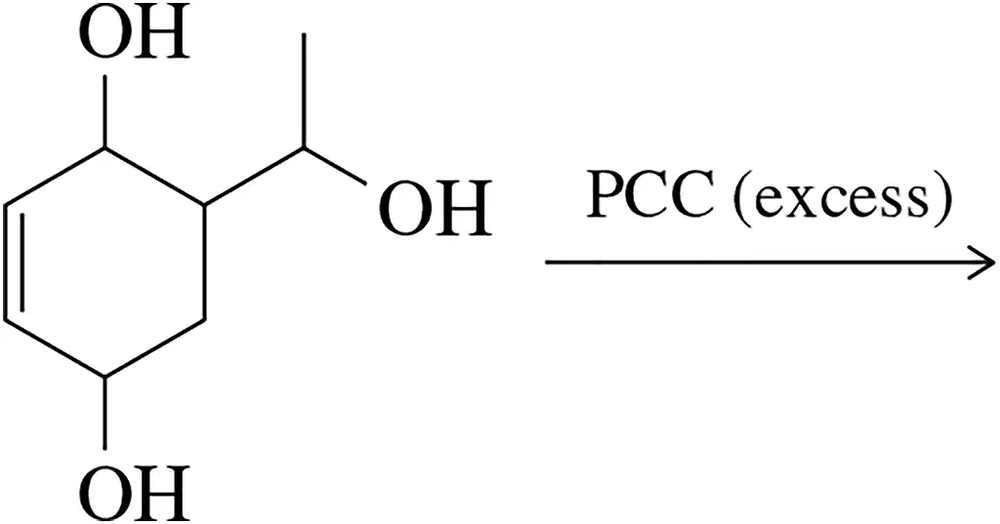
Identify the aromatic product that would result from the oxidation of the triol with an excess of PCC.
Problem 55
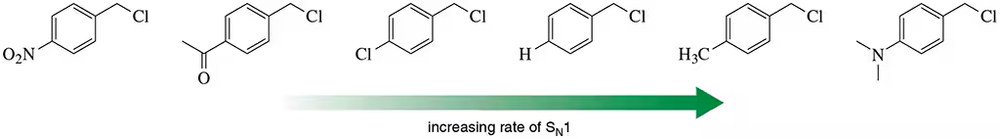
Rationalize the ranking of increasing reaction rate of the benzylic halides shown.
Problem 56
In light of your answers to Assessments 24.54 and 24.55, rank the following based on the rate of protonation of the alkene (1 = most basic, 6 = least basic). [Ignore the fact that the alkene may not be the most basic site in the molecule.]
Problem 57a
Phenol oxidation can be coupled with other reactions to form new C―C bonds using reactions studied previously. Predict the product of the following series of reactions.
(a)
Problem 57b
Phenol oxidation can be coupled with other reactions to form new C―C bonds using reactions studied previously. Predict the product of the following series of reactions.
(b)
Problem 58
Lawsone, found in the extract of henna, reacts with amino acid residues to give the characteristic color associated with henna tattoos. Suggest a mechanism for the reaction shown.
Problem 59
Provide the reagents necessary to carry out the following synthesis. What is the purpose of steps (a) and (e)?
Problem 60
Sulpiride is an antipsychotic. Provide the reagents (a) and (b) used to complete the synthesis.