 Back
BackProblem 17
The combustion of alkanes is exothermic (∆H° < 0) . Would you expect the combustion of butane or cyclobutane to be more exothermic?
Problem 18a
For each of the following processes, indicate whether you expect ∆S° to be greater than, less than, or equal to 0. Explain your answer.
(a) Boiling water
Problem 18b
For each of the following processes, indicate whether you expect ∆S° to be greater than, less than, or equal to 0. Explain your answer.
(b) 2 C6H14(l) + 19 O2(g) → 12 CO2(g) + 14 H2O(g)
Problem 18c
For each of the following processes, indicate whether you expect ∆S° to be greater than, less than, or equal to 0. Explain your answer.
(c)
Problem 18d
For each of the following processes, indicate whether you expect ∆S° to be greater than, less than, or equal to 0. Explain your answer.
(d)
Problem 19
If the following reaction is favorable, what can we say about the sign of ∆H°? Explain your answer.
Problem 20a
Draw a reaction coordinate diagram for a one step reaction that has the following values of Ea and ∆H. (a) Ea = 9 kcal/mol ; ∆H° = + 4 kcal/mol
Problem 20b
Draw a reaction coordinate diagram for a one step reaction that has the following values of Ea and ∆H. (b) Ea = 2 kcal/mol ; ∆H° = -17 kcal/mol
Problem 20c
Draw a reaction coordinate diagram for a one step reaction that has the following values of Ea and ∆H. (c) Ea = 5 kcal/mol; ∆H° = 0 kcal/mol
Problem 24a
For the following acid–base reaction, (a) identify the acid and conjugate acid
Problem 24b
For the following acid–base reaction, (b) calculate the equilibrium constant.
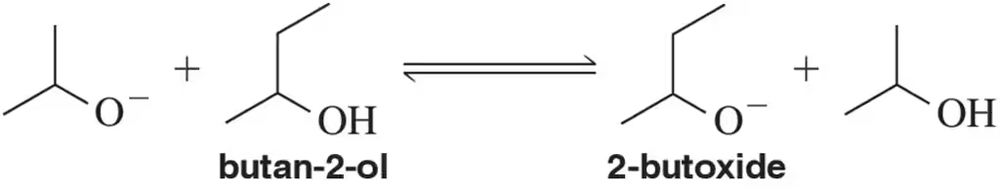
Problem 24c
For the following acid–base reaction, (c) calculate the ratio of butan-2-ol to 2-butoxide.
Problem 24d
For the following acid–base reaction, (d) calculate ∆G° at 298 K.
Problem 24e
For the following acid–base reaction, (e) calculate ∆G° at 273 K.
Problem 24f
For the following acid–base reaction, (f) calculate ∆G° at 373 K.
Problem 25a
Calculate ∆G°, ∆H°, and ∆S° for the following acid–base reactions. Rationalize the value of ∆H° based on the structure of the conjugate bases. [Assume T = 298 K.]
(a)
Problem 25b
Calculate ∆G°, ∆H°, and ∆S° for the following acid–base reactions. Rationalize the value of ∆H° based on the structure of the conjugate bases. [Assume T = 298 K.]
(b)
Problem 25c
Calculate ∆G°, ∆H°, and ∆S° for the following acid–base reactions. Rationalize the value of ∆H° based on the structure of the conjugate bases. [Assume T = 298 K.]
(c)
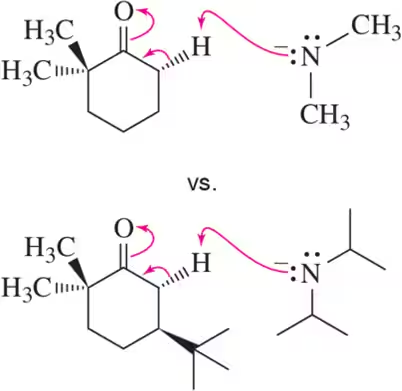
Problem 26a
Within the following pairs, pick which reaction you would expect to be faster based on having a higher value of the frequency factor (A).
(a)
Problem 26b
Within the following pairs, pick which reaction you would expect to be faster based on having a higher value of the frequency factor (A).
(b)
Problem 27
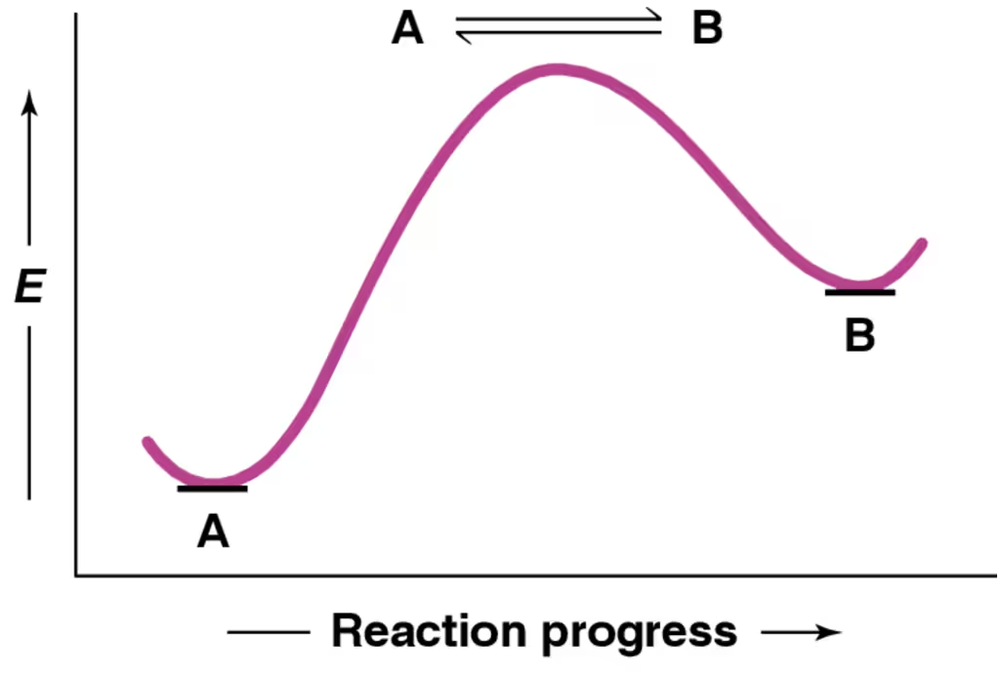
For the reaction coordinate diagram shown, is the forward or reverse reaction faster?
Problem 29a
For the following acid–base reactions studied in Assessment 5.25, draw a likely transition state. Be sure to indicate in your drawing the degree to which bonds are broken or formed.
(a)
Problem 29b
For the following acid–base reactions studied in Assessment 5.25, draw a likely transition state. Be sure to indicate in your drawing the degree to which bonds are broken or formed.
(b)
Problem 29c
For the following acid–base reactions studied in Assessment 5.25, draw a likely transition state. Be sure to indicate in your drawing the degree to which bonds are broken or formed.
(c) H3O+ + Br– ⇌ H2O + HBr
Problem 31a
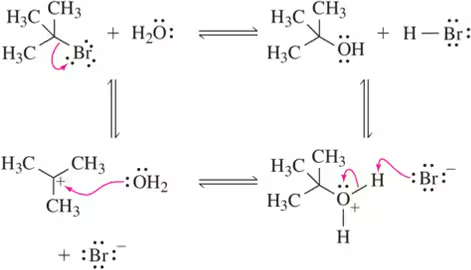
We discuss the following reactions in subsequent chapters. Given the mechanisms shown, draw the mechanism of the reverse reaction.
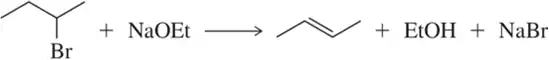
(a)
Problem 31b
We discuss the following reactions in subsequent chapters. Given the mechanisms shown, draw the mechanism of the reverse reaction.
(b)
Problem 32
Assuming that ∆H° = -15kcal/mol for the reaction in Assessment 5.31(b), show the transition state for the forward and reverse reactions.
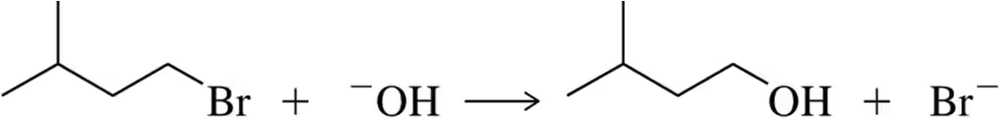
Problem 34
Write the rate law for the following reaction and identify which molecules are present in the rate-determining step. Draw a possible transition state and propose a mechanism.
Problem 35
All things being equal, would you expect a first-order reaction to be faster or slower than a second-order reaction?
Problem 36
Third-order reactions are rare. Why do you think that is?


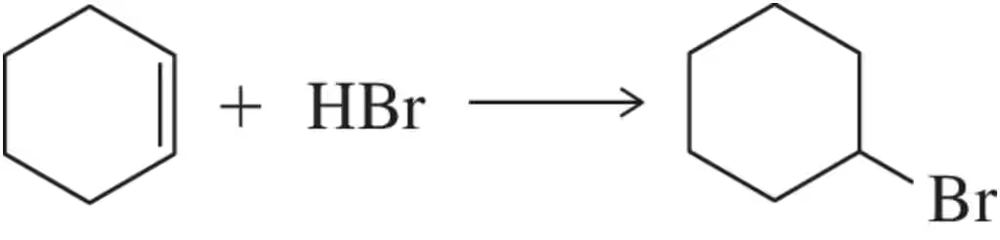







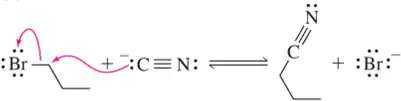
![Table displaying concentrations of [RBr] and [HO-] with corresponding reaction rates for control and trials.](https://static.studychannel.pearsonprd.tech/courses/organic-chemistry/thumbnails/1c9e5ad1-b4d8-44b4-9933-02c7b65962db)