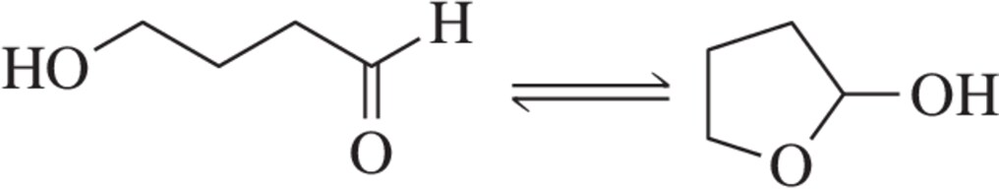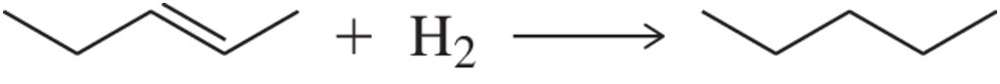Back
BackProblem 51a
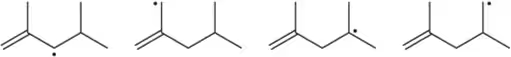
For each set of reactive intermediates, rank them in order of reactivity (1 = most reactive).
(a)
Problem 51b
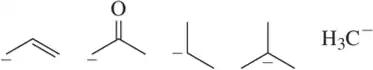
For each set of reactive intermediates, rank them in order of reactivity (1 = most reactive).
(b)
Problem 51c
For each set of reactive intermediates, rank them in order of reactivity (1 = most reactive).
(c)
Problem 52b
Predict the product(s) of the following halogenation reactions. Only one equivalent of the halogen is used in each case. If the reaction proceeds selectively, indicate this by only drawing the major product. If the reaction is not selective, draw all possible products.
(b)
Problem 52e
Predict the product(s) of the following halogenation reactions. Only one equivalent of the halogen is used in each case. If the reaction proceeds selectively, indicate this by only drawing the major product. If the reaction is not selective, draw all possible products.
(e)
Problem 52f
Predict the product(s) of the following halogenation reactions. Only one equivalent of the halogen is used in each case. If the reaction proceeds selectively, indicate this by only drawing the major product. If the reaction is not selective, draw all possible products.
(f)
Problem 53a
For each of the reaction coordinate diagrams shown, (i) indicate the number of steps in the reaction, (ii) label the intermediates, (iii) identify the rate-determining step, and (iv) tell whether Keq is greater than, less than, or equal to zero.
(a)
Problem 54a
Draw a reaction coordinate diagram, making sure to label reactants (R), products (P), intermediates (I), transition states (‡), activation energies ( Ea) , and ∆G°, for each of the following.
(a) an exothermic, one-step reaction
Problem 54b
Draw a reaction coordinate diagram, making sure to label reactants (R), products (P), intermediates (I), transition states (‡), activation energies ( Ea) , and ∆G°, for each of the following.
(b) an exothermic, two-step reaction where the second step is rate-determining
Problem 54c
Draw a reaction coordinate diagram, making sure to label reactants (R), products (P), intermediates (I), transition states (‡), activation energies ( Ea) , and ∆G°, for each of the following.
(c) a slightly endothermic, three-step reaction where the first step is rate-determining
Problem 54d
Draw a reaction coordinate diagram, making sure to label reactants (R), products (P), intermediates (I), transition states (‡), activation energies ( Ea) , and ∆G°, for each of the following.
(d) a slightly exothermic, three-step reaction where the third step is rate-determining.
Problem 56
Calculate ∆H° for the following alkene addition reaction, one we discuss further in Chapter 7. Predict the sign of ∆S° . (The BDE for C―C π bond is approximately 65 kcal/mol.)
Problem 57b
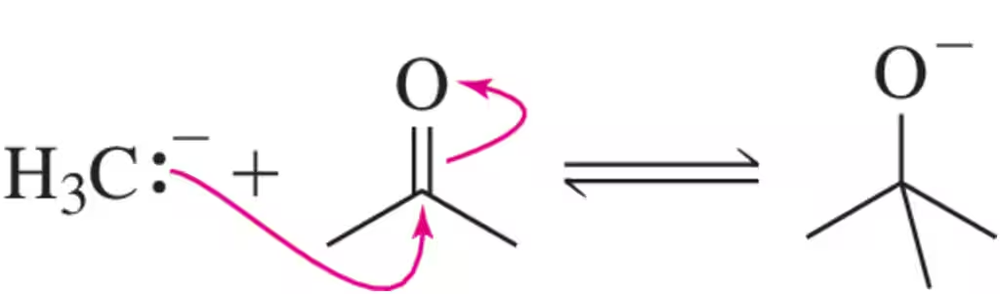
(b) Mechanistically, the reaction occurs as shown below. Why is this reaction favored? Based on the stability of the anions, estimate Keq.
Problem 59b
Reactions (a) and (b) are disfavored overall (∆G° > 0), yet they are favored based on ∆H°. Identify the bonds formed and broken for (a) and (b).
(b)
Problem 59c
Reaction (c), on the other hand, is favored (∆G° < 0). Identify the bonds formed and broken and explain this result in light of (a) and (b).
(c)
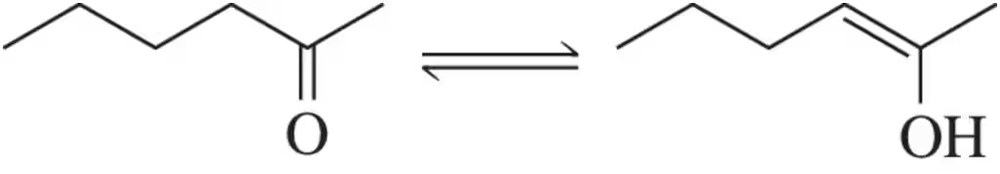
Problem 60
Keto–enol tautomerism is a reaction we discuss in detail in Chapter 19. Estimate the equilibrium constant of this reaction (BDE for C―C π bond = 65 kcal/mol ; for C―O π bond = 85 kcal/mol).
Problem 61b
The hydrogenation of alkenes is a reaction we study in Chapter 9.
(b) Is this reaction favored or disfavored in terms of entropy?
Problem 63b
Calculate Keq for the following acid–base reaction.
Problem 63c
Parts (a)–(d) of this assessment assist in the development of what will become a common theme in organic reactions and should be worked in order. [Think carefully about how each question relates to the others.]
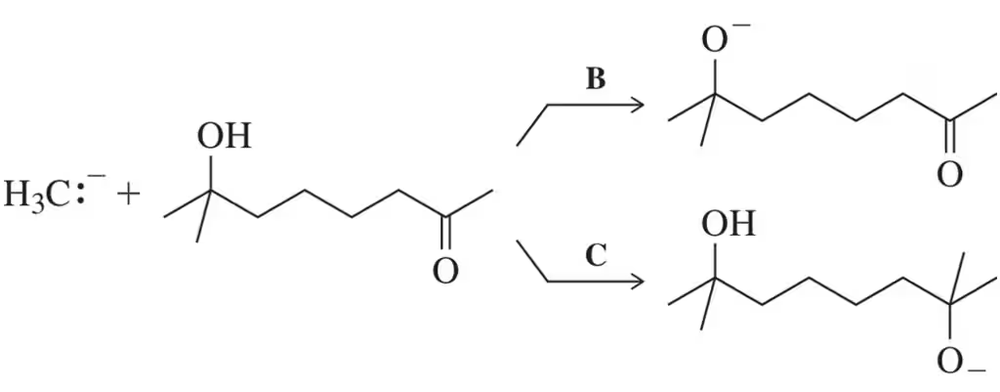
(c) Without worrying about the mechanism of the reaction, estimate an equilibrium constant for the following carbonyl addition reaction based on the relative stability of the Lewis bases.
Problem 63d
In light of your answers to parts (b) and (c), where both were shown to be quite favorable, imagine a scenario where either reaction is possible. Of the two, which would you expect to be faster? Which would you expect to be more favored? Explain each in the context of the important thermodynamic and/or kinetic parameters.
Problem 65a
In Chapter 13, we discuss the ring-opening reactions of epoxides, such as the one shown here.
(a) Based on the bonds formed and the bonds broken, calculate ∆H°.
Problem 65b
In Chapter 13, we discuss the ring-opening reactions of epoxides, such as the one shown here.
(b) Predict the sign of ∆S°.
Problem 67
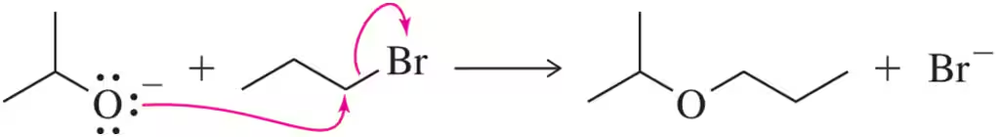
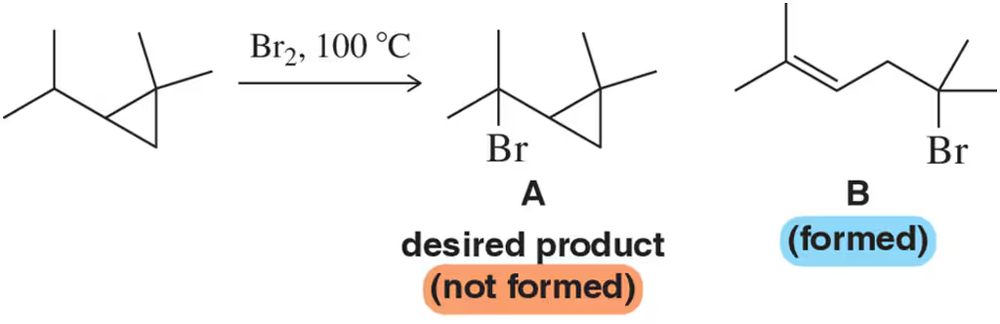
When a student attempted a bromination to produce compound A, they generated compound B instead. Rationalize the formation of B using the arrow-pushing formalism.
Problem 71
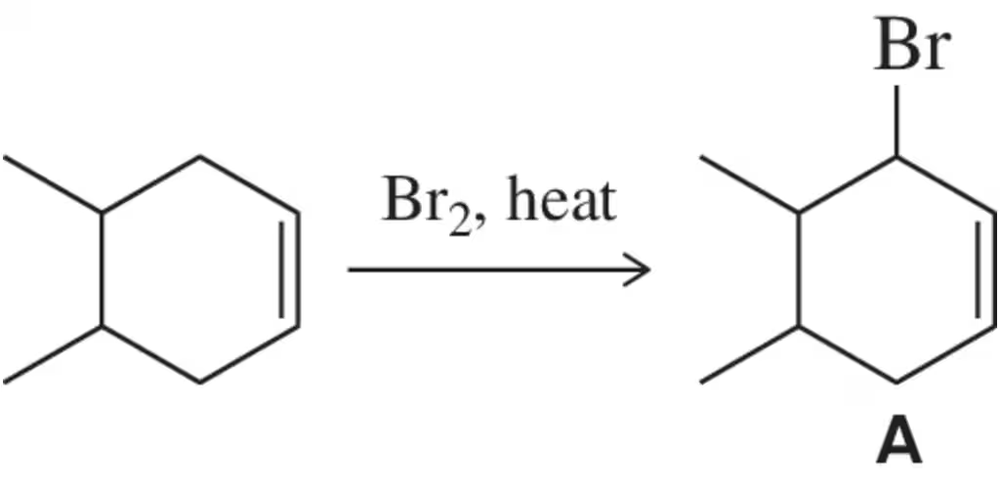
The halogenation of an alkane when there is an alkene present in the molecule does not proceed with the regioselectivity you might expect. Using principles similar to those developed in this chapter, rationalize the formation of A as the only product. We study this reaction further in Chapter 8.
Problem 72a
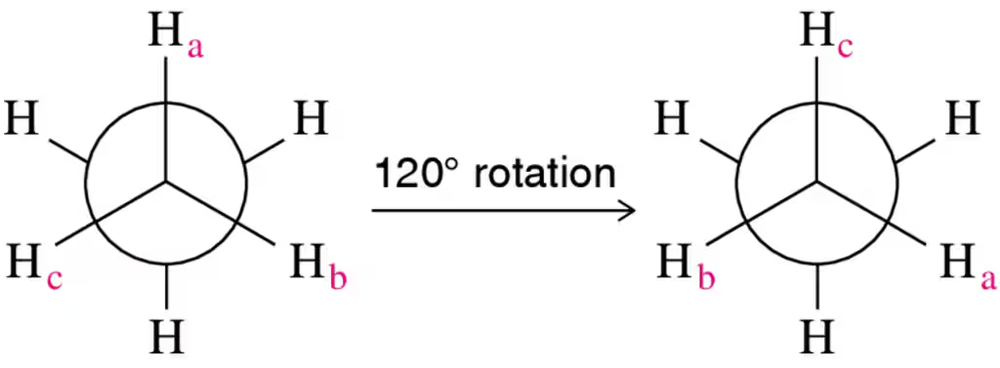
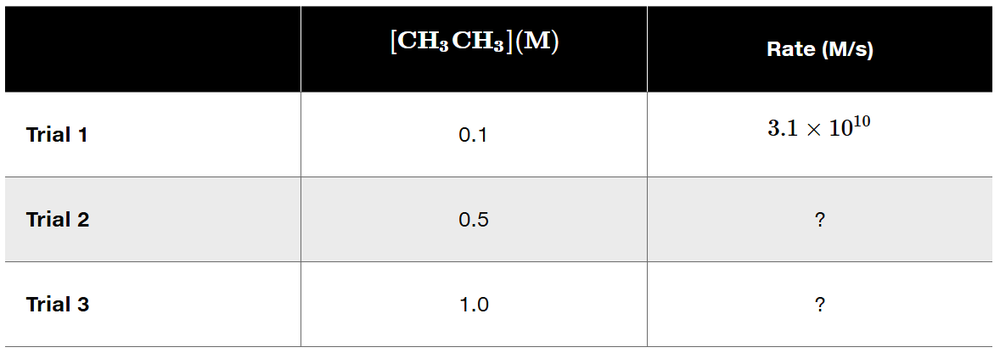
Parts (a)–(f) of this assessment refer to the rotation around the single bond of ethane.
(a) Given that the rate of the reaction is independent of concentration, fill in the missing rates in the following table.
Problem 72b
Parts (a)–(f) of this assessment refer to the rotation around the single bond of ethane.
(b) What is the order of the reaction with regard to ethane?
Problem 72c
Parts (a)–(f) of this assessment refer to the rotation around the single bond of ethane.
(c) Write the rate law for this reaction.
Problem 73
For rotation around a bond, the rate constant is equal to the reaction rate. Why?