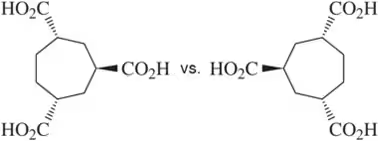Back
BackProblem 32
Imagine a sample that is enriched in the R enantiomer. If the % ee of the sample is 83%,
(a) what percent of the mixture is racemic?
(b) What is the ratio of R to S?
Problem 33
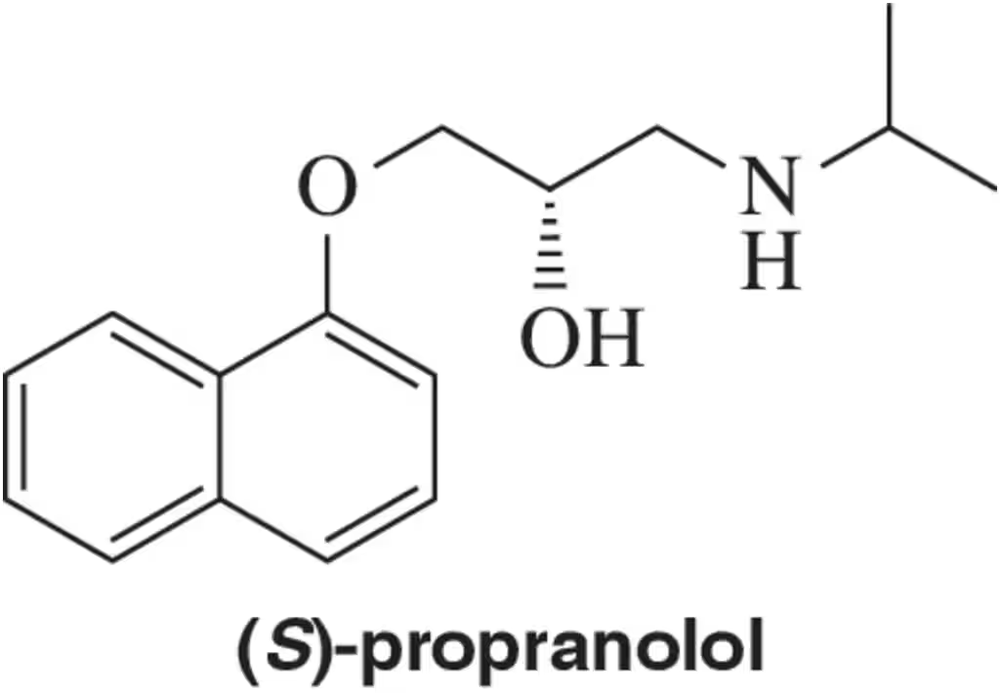
(S)-Propranolol, a drug used for the treatment of anxiety, has a specific rotation of -25.5° . Attempting to prepare it in enantiopure form, a chemist produced a compound that gave a specific rotation of -18.3° . What is the ratio of (S)- to (R)-propranolol produced by the chemist?
Problem 34
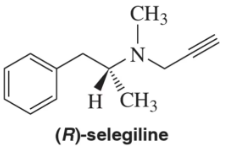
(R)-Selegiline, a monoamine oxidase (MAO) inactivator, was approved by the FDA in 1989 for the treatment of Parkinson's disease. In pure form, it has a specific rotation, [α]²⁰D = - 11.0°. What is the expected specific rotation of a mixture containing 64% S and 36% R?
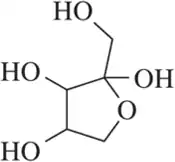
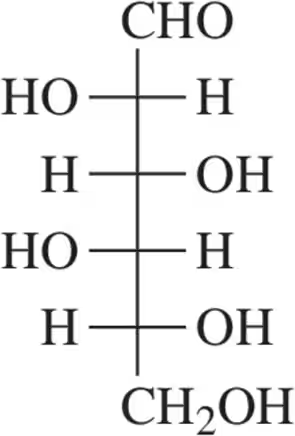
Problem 35a
For the molecules shown,
(i) count the number of stereocenters present and
(ii) draw all possible stereoisomers.
(iii) Identify the relationships between stereoisomers as enantiomers or diastereomers.
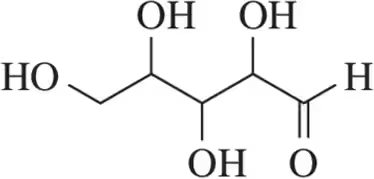
(a)
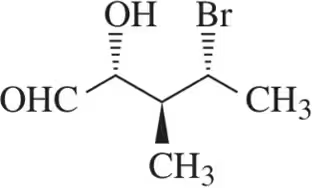
Problem 35b
For the molecules shown,
(i) count the number of stereocenters present and
(ii) draw all possible stereoisomers.
(iii) Identify the relationships between stereoisomers as enantiomers or diastereomers.
(b)
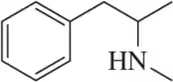
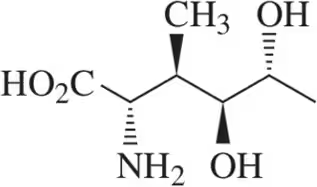
Problem 35c
For the molecules shown,
(i) count the number of stereocenters present and
(ii) draw all possible stereoisomers.
(iii) Identify the relationships between stereoisomers as enantiomers or diastereomers.
(c)
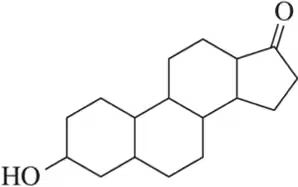
Problem 36a
How many stereoisomers are possible for each of the following molecules?
(a)
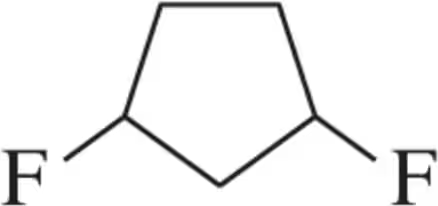
Problem 36c
How many stereoisomers are possible for each of the following molecules?
(c)
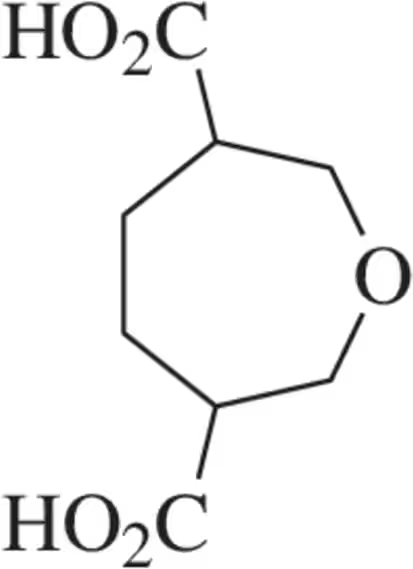
Problem 36d
How many stereoisomers are possible for each of the following molecules?
(d)
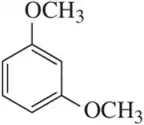
Problem 36e
How many stereoisomers are possible for each of the following molecules?
(e)
Problem 37a
Which of the following compounds are meso?
(a)
Problem 37c
Which of the following compounds are meso?
(c)
Problem 37e
Which of the following compounds are meso?
(e)
Problem 37f
Which of the following compounds are meso?
(f)
Problem 37g
Which of the following compounds are meso?
(g)
Problem 38a
How many stereoisomers are possible for each of the following molecules?
(a)
Problem 38b
How many stereoisomers are possible for each of the following molecules?
(b)
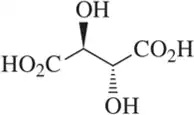
Problem 39
Which of the following diastereomers of hexane-2,5-diol is optically inactive? Why?
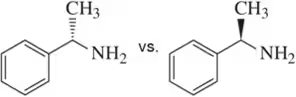
Problem 40a
(i) Which of the following pairs of compounds would you expect to have different physical properties?
(ii) What is the relationship between each of the pairs?
(iii) Assign the absolute configuration of each stereocenter to confirm your answer.
(a)
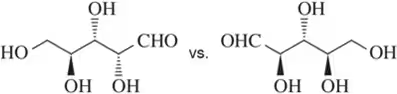
Problem 40b
(i) Which of the following pairs of compounds would you expect to have different physical properties?
(ii) What is the relationship between each of the pairs?
(iii) Assign the absolute configuration of each stereocenter to confirm your answer.
(b)
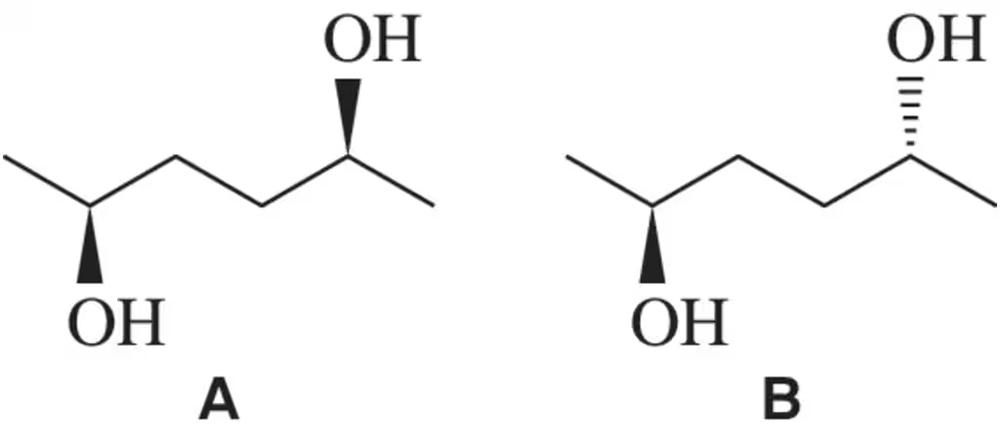
Problem 40c
(i) Which of the following pairs of compounds would you expect to have different physical properties?
(ii) What is the relationship between each of the pairs?
(iii) Assign the absolute configuration of each stereocenter to confirm your answer.
(c)
Problem 41a
Convert the Fischer projections into line-angle drawings and assign (R) and (S) at each chiral center.
(a)
Problem 41c
Convert the Fischer projections into line-angle drawings and assign (R) and (S) at each chiral center.
(c)
Problem 42a
Convert the line-angle drawings into Fischer projections.
(a)
Problem 42b
Convert the line-angle drawings into Fischer projections.
(b)
Problem 42c
Convert the line-angle drawings into Fischer projections.
(c)
Problem 43
How might you separate enantiomers of 2-phenylpropionic acid?
Problem 44
Figure 6.52 <IMAGE> shows the lipase-catalyzed kinetic resolution of secondary alcohols. Show a reaction coordinate diagram that rationalizes the results obtained.
Problem 45a
We discuss the reaction of Grignard reagents (organomagnesium compounds) to ketones in Chapter 17. Mechanistically, the reaction proceeds by the nucleophilic addition of a methyl carbanion to the electrophilic carbon of the carbonyl, breaking the C―Oπ bond, resulting in an alkoxide intermediate that is subsequently protonated to produce the 3° alcohol.
(a) Why does this reaction produce a racemic mixture of 3° alcohols?
Problem 45b
We discuss the reaction of Grignard reagents (organomagnesium compounds) to ketones in Chapter 17. Mechanistically, the reaction proceeds by the nucleophilic addition of a methyl carbanion to the electrophilic carbon of the carbonyl, breaking the C―Oπ bond, resulting in an alkoxide intermediate that is subsequently protonated to produce the 3° alcohol.
(b) Why, when the substrate is modified slightly, does the reaction result in an excess of one stereoisomer?