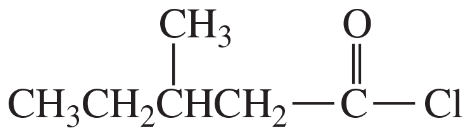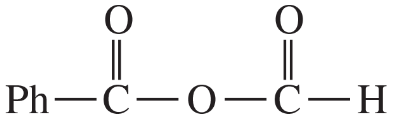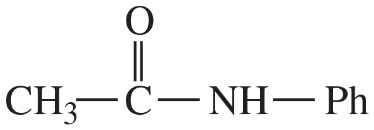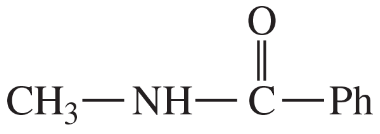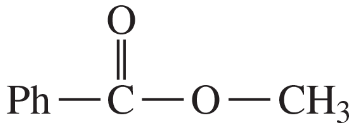Back
BackProblem 34d
Suggest the most appropriate reagent for each synthesis, and explain your choice.
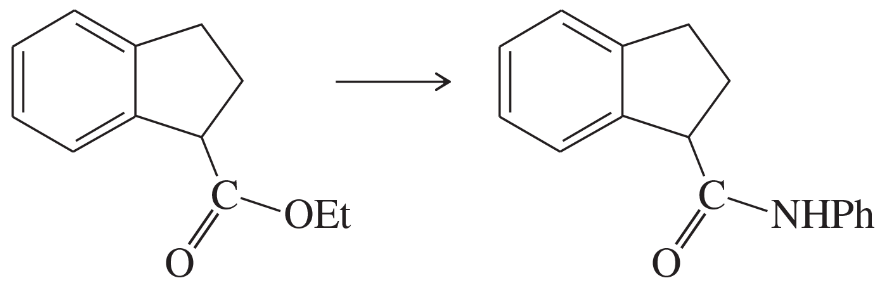
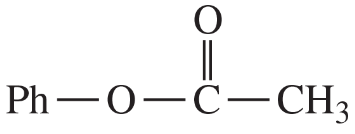
(d)
Problem 35a,b,c
Show how you would synthesize each compound, starting with an ester containing no more than eight carbon atoms. Any other necessary reagents may be used.
(a) Ph3C–OH
(b) (PhCH2)2CHOH
(c) PhCONHCH2CH3
Problem 35d,e,f
Show how you would synthesize each compound, starting with an ester containing no more than eight carbon atoms. Any other necessary reagents may be used.
(d) Ph2CHOH
(e) PhCH2OH
(f) PhCOOH
Problem 35i
Show how you would synthesize each compound, starting with an ester containing no more than eight carbon atoms. Any other necessary reagents may be used.
(i) HO–(CH2)8–OH
Problem 36a,b
Show how you would accomplish the following synthetic transformations. You may use any necessary reagents.
(a) N-ethylbenzamide → benzylethylamine
(b) ethyl benzoate → N-ethylbenzamide
Problem 36c,d
Show how you would accomplish the following synthetic transformations. You may use any necessary reagents.
(c) pyrrolidine → N-acetylpyrrolidine
(d) γ-aminobutyric acid → pyrrolidine
Problem 37
Show how you would accomplish the following syntheses using amides as intermediates. You may use any necessary reagents.
(a) benzoic acid → benzyldimethylamine
(b) pyrrolidine → N-ethylpyrrolidine
(c) cyclopentanecarboxylic acid → cyclopentanecarbonitrile
Problem 38a
Show how you would convert the following starting materials to the indicated nitriles:
(a) phenylacetic acid → phenylacetonitrile
Problem 38b
Show how you would convert the following starting materials to the indicated nitriles:
(b) phenylacetic acid → 3-phenylpropionitrile
Problem 38c
Show how you would convert the following starting materials to the indicated nitriles:
(c) p-chloronitrobenzene → p-chlorobenzonitrile
Problem 39a
Show how each transformation may be accomplished by using a nitrile as an intermediate. You may use any necessary reagents.
(a) hexan-1-ol → heptan-1-amine
Problem 39b
Show how each transformation may be accomplished by using a nitrile as an intermediate. You may use any necessary reagents.
(b) cyclohexanecarboxamide → cyclohexyl ethyl ketone
Problem 39c
Show how each transformation may be accomplished by using a nitrile as an intermediate. You may use any necessary reagents.
(c) octan-1-ol → decan-2-one
Problem 40
Propose a mechanism for the reaction of methyl isocyanate with 1-naphthol to give Sevin® insecticide.
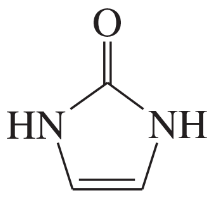
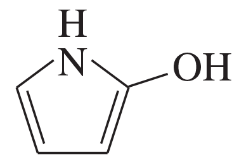
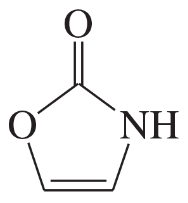
Problem 41a,b,c(i)
For each heterocyclic compound,
(i) explain what type of acid derivative is present.
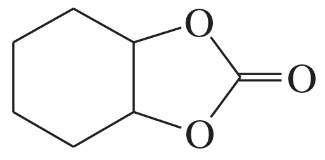
(a)
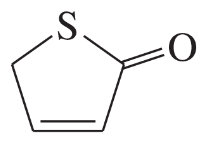
(b)
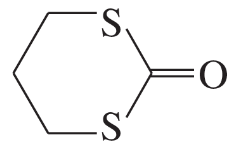
(c)
Problem 41a,b,c(ii)
For each heterocyclic compound,
(ii) show what compounds would result from complete hydrolysis.
(a)
(b)
(c)
Problem 41a,b,c(iii)
For each heterocyclic compound,
(iii) are any of the rings aromatic? Explain.
(a)
(b)
(c)
Problem 41d
For each heterocyclic compound,
(i) explain what type of acid derivative is present.
(ii) show what compounds would result from complete hydrolysis.
(iii) are any of the rings aromatic? Explain.
(d)
Problem 41e
For each heterocyclic compound,
(i) explain what type of acid derivative is present.
(ii) show what compounds would result from complete hydrolysis.
(iii) are any of the rings aromatic? Explain.
(e)
Problem 41f
For each heterocyclic compound,
(i) explain what type of acid derivative is present.
(ii) show what compounds would result from complete hydrolysis.
(iii) are any of the rings aromatic? Explain.
(f)
Problem 42a,b,c
Draw structures to correspond with the following common and systematic names:
(a) phenyl formate
(b) cyclohexyl benzoate
(c) cyclopentyl phenylacetate
Problem 42d,e
Draw structures to correspond with the following common and systematic names:
(d) N-butylacetamide
(e) N,N-dimethylformamide
Problem 42f,g
Draw structures to correspond with the following common and systematic names:
(f) benzoic propionic anhydride
(g) benzamide
Problem 42h
Draw structures to correspond with the following common and systematic names:
(h) γ-hydroxyvaleronitrile
Problem 42k,l
Draw structures to correspond with the following common and systematic names:
(k) phenyl isocyanate
(l) cyclobutyl ethyl carbonate
Problem 42m
Draw structures to correspond with the following common and systematic names:
(m) δ-caprolactam
Problem 42n,o
Draw structures to correspond with the following common and systematic names:
(n) trichloroacetic anhydride
(o) ethyl N-methyl carbamate
Problem 43a,b
Give appropriate names for the following compounds:
(a)
(b)
Problem 43c,d
Give appropriate names for the following compounds:
(c)
(d)
Problem 43e,f
Give appropriate names for the following compounds:
(e)
(f)