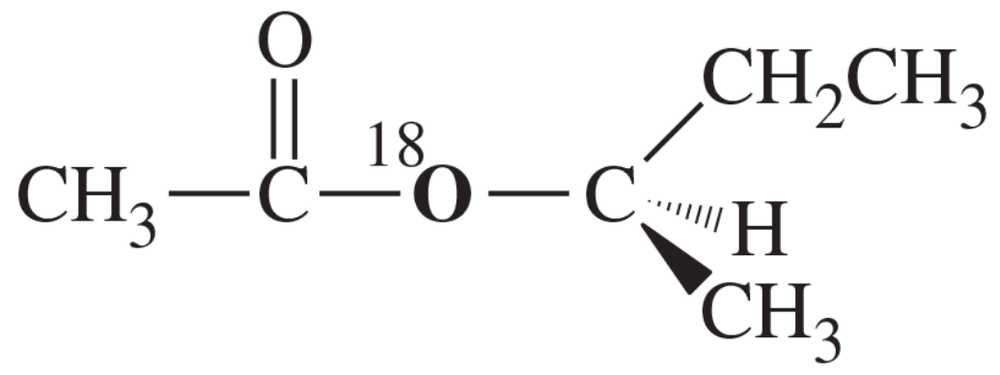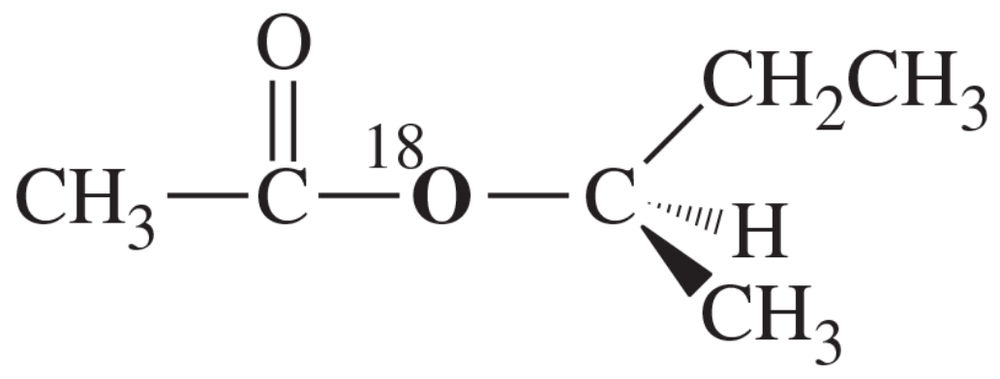Back
BackProblem 14
Propose a mechanism for the acid-catalyzed hydrolysis of phenylalanine ethyl ester.
Problem 14a
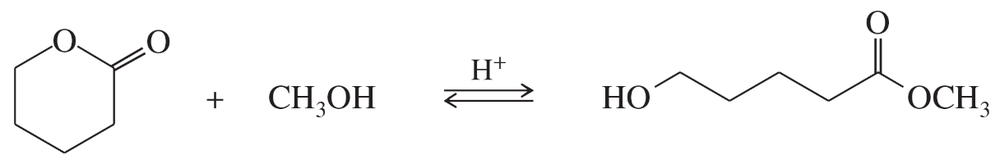
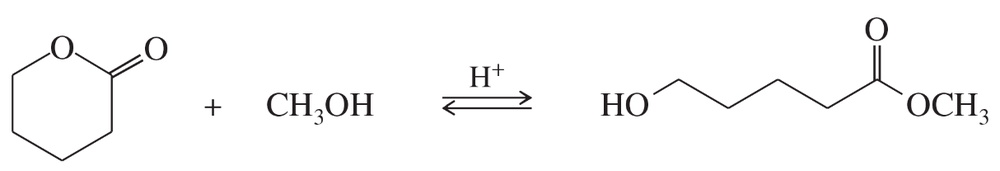
Propose a mechanism for the following ring-opening transesterification. Use the mechanism in Problem 21-13 as a model.
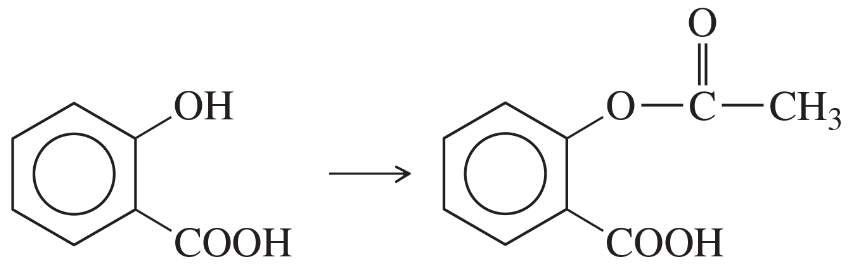
Problem 15
1. Propose a mechanism for the acid-catalyzed reaction of salicylic acid with acetic anhydride.
2. Explain why a single drop of sulfuric acid dramatically increases the reaction rate.
Problem 16a
Suppose we have some optically pure (R)-2-butyl acetate that has been 'labeled' with the heavy 18O isotope at one oxygen atom as shown.
(a) Draw a mechanism for the hydrolysis of this compound under basic conditions. Predict which of the products will contain the 18O label. Also predict whether the butan-2-ol product will be pure (R), pure (S), or racemized.
Problem 16b
Suppose we have some optically pure (R)-2-butyl acetate that has been "labeled" with the heavy 18O isotope at one oxygen atom as shown.
(b) Repeat part (a) for the acid-catalyzed hydrolysis of this compound.
Problem 16c
Suppose we have some optically pure (R)-2-butyl acetate that has been 'labeled' with the heavy 18O isotope at one oxygen atom as shown.
(c) Explain how you would prove experimentally where the 18O label appears in the products. (18O is not radioactive.)
Problem 17a
Explain why we speak of acidic hydrolysis of an ester as acid-catalyzed, but of basic hydrolysis as base-promoted.
Problem 17b
Soap manufacturers always use base to hydrolyze fats, and never acid. Suggest two reasons that basic hydrolysis is preferred.
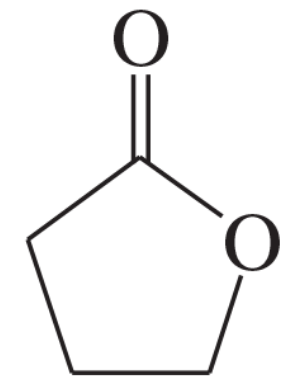
Problem 18
Propose a mechanism for the base-promoted hydrolysis of γ-butyrolactone:
Problem 19
Draw the important resonance contributors for both resonance-stabilized cations (in brackets) in the mechanism for acid-catalyzed hydrolysis of an amide.
Problem 20a
Propose a mechanism for the hydrolysis of N,N-dimethylacetamide
(a) under basic conditions.
Problem 20b
Propose a mechanism for the hydrolysis of N,N-dimethylacetamide
(b) under acidic conditions.
Problem 20.21
Show how the following ketones might be synthesized from the indicated acids, using any necessary reagents.
(a) propiophenone from propionic acid (using Friedel–Crafts acylation)
Problem 22
Propose a mechanism for the basic hydrolysis of benzonitrile to the benzoate ion and ammonia.
Problem 23
The mechanism for acidic hydrolysis of a nitrile resembles the basic hydrolysis, except that the nitrile is first protonated, activating it toward attack by a weak nucleophile (water). Under acidic conditions, the proton transfer (tautomerism) involves protonation on nitrogen followed by deprotonation on oxygen. Propose a mechanism for the acid-catalyzed hydrolysis of benzonitrile to benzamide.
Problem 24a
In which step(s) of the hydride reduction of an ester does the compound undergo reduction? (Hint: Count the bonds to oxygen.)
Problem 24b
Propose a mechanism for the reduction of octanoyl chloride by lithium aluminum hydride.
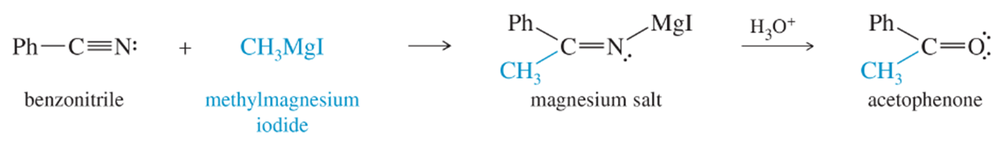
Problem 26
Draw a mechanism for the acidic hydrolysis of the magnesium salt shown below to acetophenone.
Problem 27
Draw a mechanism for the reaction of propanoyl chloride with 2 moles of phenylmagnesium bromide.
Problem 28a
Show how you would add a Grignard reagent to an ester or a nitrile to synthesize
(a) 4-phenylheptan-4-ol.
Problem 28b
Show how you would add a Grignard reagent to an ester or a nitrile to synthesize
(b) heptan-4-ol.
Problem 28c
Show how you would add a Grignard reagent to an ester or a nitrile to synthesize
(c) pentan-2-one.
Problem 30a
Show how Friedel–Crafts acylation might be used to synthesize the following compounds.
a. acetophenone
Problem 30b
Show how Friedel–Crafts acylation might be used to synthesize the following compounds.
b. benzophenone
Problem 30c
Show how Friedel–Crafts acylation might be used to synthesize the following compounds.
c. n-butylbenzene
Problem 32a,b
Show how you would use anhydrides to synthesize the following compounds. In each case, explain why an anhydride might be preferable to an acid chloride.
(a) n-octyl formate
(b) n-octyl acetate
Problem 32c,d
Show how you would use anhydrides to synthesize the following compounds. In each case, explain why an anhydride might be preferable to an acid chloride.
(c) phthalic acid monoamide
(d) succinic acid monomethyl ester
Problem 34a
Suggest the most appropriate reagent for each synthesis, and explain your choice.
(a)
Problem 34b
Suggest the most appropriate reagent for each synthesis, and explain your choice.
(b)
Problem 34c
Suggest the most appropriate reagent for each synthesis, and explain your choice.
(c)