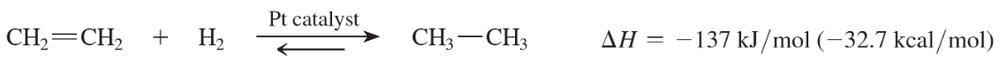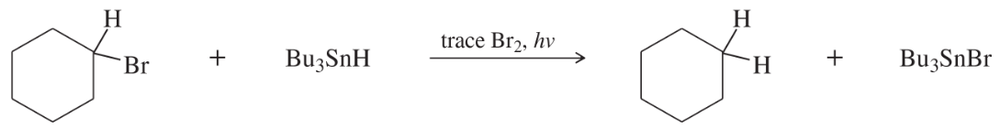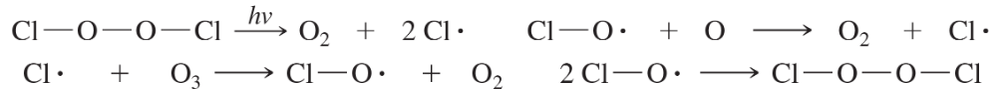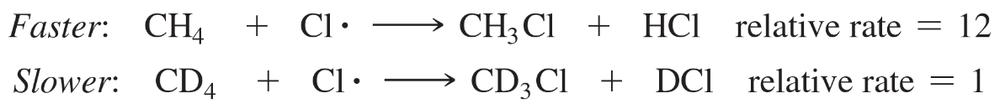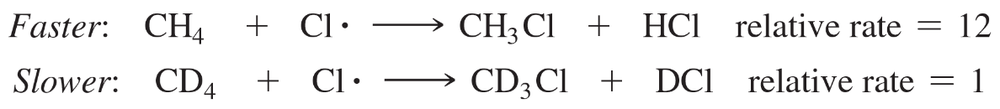Back
BackProblem 52
When dichloromethane is treated with strong NaOH, an intermediate is generated that reacts like a carbene. Draw the structure of this reactive intermediate, and propose a mechanism for its formation.
Problem 53
When ethene is treated in a calorimeter with H2 and a Pt catalyst, the heat of reaction is found to be –137 kJ/mol (–32.7 kcal/mol), and the reaction goes to completion. When the reaction takes place at 1400 K, the equilibrium is found to be evenly balanced, with Keq =1. Compute the value of ΔS for this reaction.
Problem 54
When a small amount of iodine is added to a mixture of chlorine and methane, it prevents chlorination from occurring. Therefore, iodine is a free-radical inhibitor for this reaction. Calculate ΔH° values for the possible reactions of iodine with species present in the chlorination of methane, and use these values to explain why iodine inhibits the reaction. (The I―Cl bond-dissociation enthalpy is 211 kJ/mol or 50 kcal/mol.)
Problem 55b
Tributyltin hydride (Bu3SnH) is used synthetically to reduce alkyl halides, replacing a halogen atom with hydrogen. Free-radical initiators promote this reaction, and free-radical inhibitors are known to slow or stop it. Your job is to develop a mechanism, using the following reaction as the example.
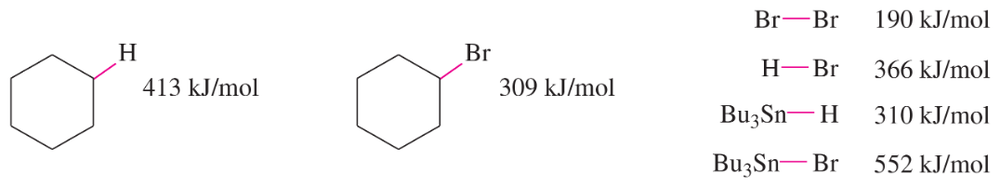
The following bond-dissociation enthalpies may be helpful:
b. Calculate values of ΔH for your proposed steps to show that they are energetically feasible. (Hint: A trace of Br2 and light suggests it’s there only as an initiator, to create Br• radicals. Then decide which atom can be abstracted most favorably from the starting materials by the Br• radical. That should complete the initiation. Finally, decide what energetically favored propagation steps will accomplish the reaction.)
Problem 56
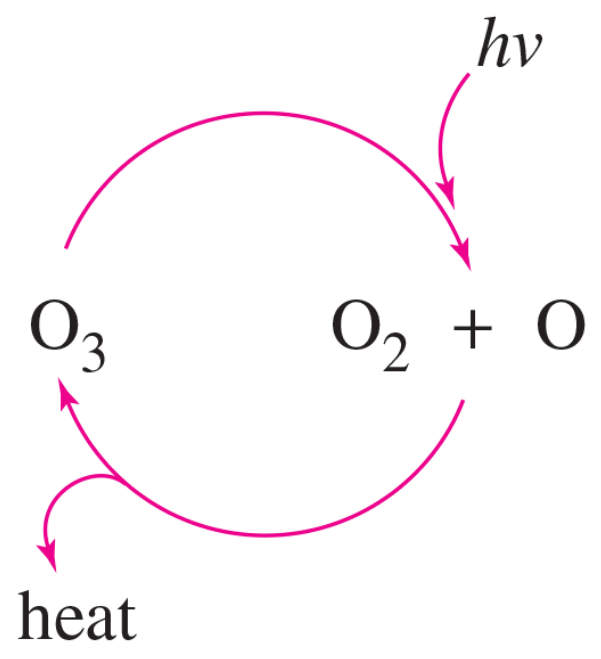
When healthy, Earth’s stratosphere contains a low concentration of ozone (O3) that absorbs potentially harmful ultraviolet (UV) radiation by the cycle shown at right.
Chlorofluorocarbon refrigerants, such as Freon 12 (CF2Cl2), are stable in the lower atmosphere, but in the stratosphere they absorb high-energy UV radiation to generate chlorine radicals.
The presence of a small number of chlorine radicals appears to lower ozone concentrations dramatically. The following reactions are all known to be exothermic (except the one requiring light) and to have high rate constants. Propose two mechanisms to explain how a small number of chlorine radicals can destroy large numbers of ozone molecules. Which of the two mechanisms is more likely when the concentration of chlorine atoms is very small?
Problem 57a
Deuterium (D) is the hydrogen isotope of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C―D bond is slightly stronger than the C―H bond by 5.0 kJ/mol (1.2 kcal/mol). Reaction rates tend to be slower when a C―D bond (as opposed to a C―H bond) is broken in a rate-limiting step.
This effect, called a kinetic isotope effect, is clearly seen in the chlorination of methane. Methane undergoes free-radical chlorination 12 times as fast as tetradeuteriomethane (CD4).
a. Draw the transition state for the rate-limiting step of each of these reactions, showing how a bond to hydrogen or deuterium is being broken in this step.
Problem 57b
Deuterium (D) is the hydrogen isotope of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C―D bond is slightly stronger than the C―H bond by 5.0 kJ/mol (1.2 kcal/mol). Reaction rates tend to be slower when a C―D bond (as opposed to a C―H bond) is broken in a rate-limiting step.
This effect, called a kinetic isotope effect, is clearly seen in the chlorination of methane. Methane undergoes free-radical chlorination 12 times as fast as tetradeuteriomethane (CD4).
b. Monochlorination of deuterioethane (C2H5D) leads to a mixture containing 93% C2H4DCl and 7% C2H5Cl. Calculate the relative rates of abstraction per hydrogen and deuterium in the chlorination of deuterioethane.
Problem 57c
Deuterium (D) is the hydrogen isotope of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C―D bond is slightly stronger than the C―H bond by 5.0 kJ/mol (1.2 kcal/ mol). Reaction rates tend to be slower when a C―D bond (as opposed to a C―H bond) is broken in a rate-limiting step.
This effect, called a kinetic isotope effect, is clearly seen in the chlorination of methane. Methane undergoes free-radical chlorination 12 times as fast as tetradeuteriomethane (CD4).
c. Consider the thermodynamics of the chlorination of methane and the chlorination of ethane, and use the Hammond postulate to explain why one of these reactions has a much larger isotope effect than the other.
Problem 58
Iodination of alkanes using iodine (I2) is usually an unfavorable reaction. (See Problem 4-17 , for example.) Tetraiodomethane (CI4) can be used as the iodine source for iodination in the presence of a free-radical initiator such as hydrogen peroxide. Propose a mechanism (involving mildly exothermic propagation steps) for the following proposed reaction. Calculate the value of ΔH for each of the steps in your proposed mechanism.
The following bond-dissociation energies maybe helpful: