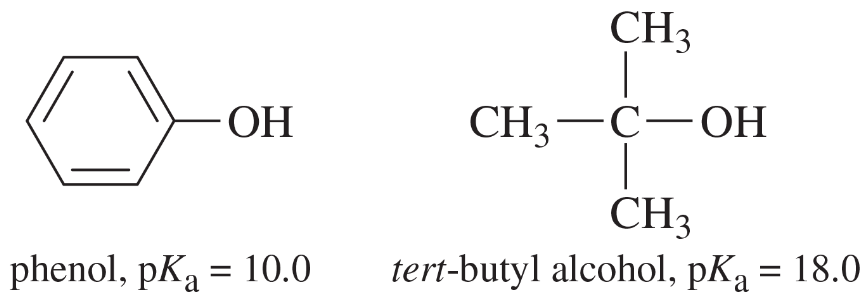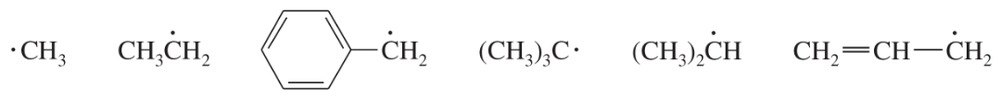Back
BackProblem 38
a. Draw an approximate reaction-energy diagram for the acid–base reaction of phenol (see below) with 1-molar aqueous sodium hydroxide solution.
b. On the same diagram, draw an approximate reaction-energy diagram for the acid–base reaction of tert-butyl alcohol (see below) with 1-molar aqueous sodium hydroxide solution.
Problem 39
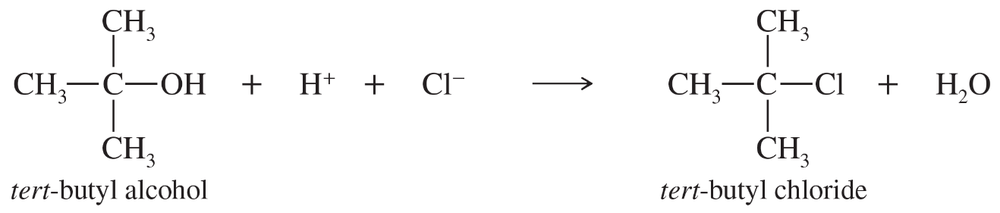
Treatment of tert-butyl alcohol with concentrated HCl gives tert-butyl chloride.
When the concentration of H+ is doubled, the reaction rate doubles. When the concentration of tert-butyl alcohol is tripled, the reaction rate triples. When the chloride ion concentration is quadrupled, however, the reaction rate is unchanged. Write the rate equation for this reaction.
Problem 40a,b
Label each hydrogen atom in the following compounds as primary (1°), secondary (2°), or tertiary (3°).
(a) CH3CH2CH(CH3)2
(b) (CH3)3CCH2CH3
Problem 40c,d
Label each hydrogen atom in the following compounds as primary (1°), secondary (2°), or tertiary (3°).
(c) (CH3)2CHCH(CH3)CH2CH3
(d)
Problem 40e,f
Label each hydrogen atom in the following compounds as primary (1°), secondary (2°), or tertiary (3°).
(e)
(f)
Problem 41a
Use bond-dissociation enthalpies (Table 4-2, p. 167) to calculate values of ΔH° for the following reactions.
a. CH3—CH3 + I2 → CH3CH2I + HI
Problem 41b
Use bond-dissociation enthalpies (Table 4-2, p. 167) to calculate values of ΔH° for the following reactions.
b. CH3CH2Cl + HI → CH3CH2I + HCl
Problem 41c
Use bond-dissociation enthalpies (Table 4-2, p. 167) to calculate values of ΔH° for the following reactions.
c. (CH3)3C—OH + HCl → (CH3)3C—Cl + H2O
Problem 41d
Use bond-dissociation enthalpies (Table 4-2, p. 167) to calculate values of ΔH° for the following reactions.
d. CH3CH2CH3 + H2 → CH3CH3 + CH4
Problem 42
Use the information in Table 4-2 (p. 167) to rank the following radicals in decreasing order of stability.
Problem 43(1)c,d
For each alkane,
1. draw all the possible monochlorinated derivatives.
c. 2-methylpentane
d. 2,2,3,3-tetramethyl butane
Problem 43(3)c,d
For each alkane,
3. which monobrominated derivatives could you form in good yield by free-radical bromination?
c. 2-methylpentane
d. 2,2,3,3-tetramethylbutane
Problem 43(3)a,b
For each alkane, which monobrominated derivatives could you form in good yield by free-radical bromination?
a. Cyclopentane
b. Methylcyclopentnae
Problem 43(1)a,b
For each alkane,
1. draw all the possible monochlorinated derivatives.
a. Cyclopentane
b. Methylcyclopentane
Problem 43(2)a,b
For each alkane,
2. determine whether free-radical chlorination would be a good way to make any of these monochlorinated derivatives. Will the reaction give mostly one major product?
a. Cyclopentane
b. Methylcyclopentane
Problem 44
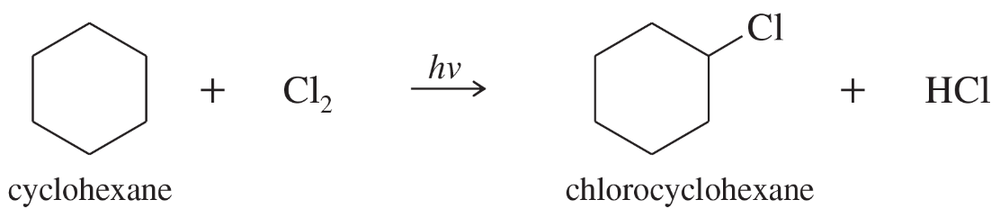
Write a mechanism for the light-initiated reaction of cyclohexane with chlorine to give chlorocyclohexane. Label the initiation and propagation steps.
Problem 45a
Draw the important resonance forms of the following free radicals.
a.
Problem 45b
Draw the important resonance forms of the following free radicals.
b.
Problem 45c,d
Draw the important resonance forms of the following free radicals.
(c)
(d)
Problem 46a
In the presence of a small amount of bromine, the following light-promoted reaction has been observed.
a. Write a mechanism for this reaction. Your mechanism should explain how both products are formed. (Hint: Notice which H atom has been lost in both products.)
Problem 46b
In the presence of a small amount of bromine, the following light-promoted reaction has been observed.
b. Explain why only this one type of hydrogen atom has been replaced, in preference to any of the other hydrogen atoms in the starting material.
Problem 47a,b
For each compound, predict the major product of free-radical bromination. Remember that bromination is highly selective, and only the most stable radical will be formed.
(a) cyclohexane
(b) methylcyclopentane
Problem 47e
For each compound, predict the major product of free-radical bromination. Remember that bromination is highly selective, and only the most stable radical will be formed.
(e)
Problem 47f
For each compound, predict the major product of free-radical bromination. Remember that bromination is highly selective, and only the most stable radical will be formed.
(f)
Problem 48a
When exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a chlorination reaction occurs. The products are found to contain substantial amounts of di-, tri-, and tetrachloromethane, as well as unreacted methane.
a. Explain how a mixture is formed from this stoichiometric mixture of reactants, and propose mechanisms for the formation of these compounds from chloromethane.
Problem 48b
When exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a chlorination reaction occurs. The products are found to contain substantial amounts of di-, tri-, and tetrachloromethane, as well as unreacted methane.
b. How would you run this reaction to get a good conversion of methane to CH3Cl? Of methane to CCl4?
Problem 49a
The chlorination of pentane gives a mixture of three monochlorinated products.
a. Draw their structures.
Problem 49b
The chlorination of pentane gives a mixture of three monochlorinated products.
b. Predict the ratios in which these monochlorination products will be formed, remembering that a chlorine atom abstracts a secondary hydrogen about 4.5 times as fast as it abstracts a primary hydrogen.
Problem 50a
a. Draw the structure of the transition state for the second propagation step in the chlorination of methane.
Show whether the transition state is product-like or reactant-like and which of the two partial bonds is stronger.
Problem 51
Peroxides are often added to free-radical reactions as initiators because the oxygen–oxygen bond cleaves homolytically rather easily. For example, the bond-dissociation enthalpy of the O―O bond in hydrogen peroxide (H―O―O―H) is only 213 kJ/mol (51 kcal/mol). Give a mechanism for the hydrogen peroxide-initiated reaction of cyclopentane with chlorine. The BDE for HO―Cl is 210 kJ/mol (50 kcal/mol).