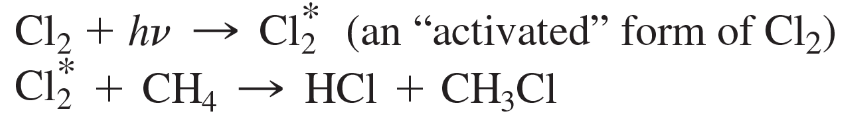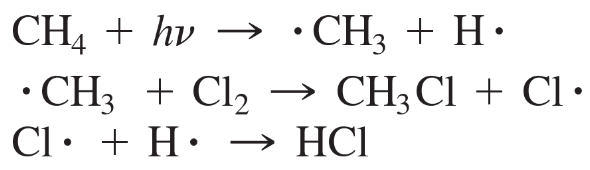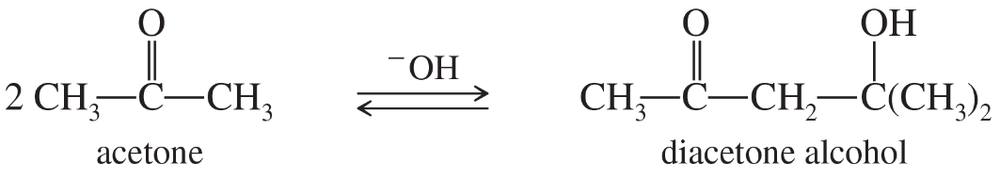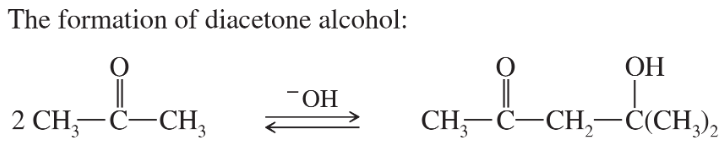Back
BackProblem 1
Draw Lewis structures for the following free radicals.
a. The ethyl radical, CH3—ĊH2
b. The tert-butyl radical, (CH3)3C•
Problem 2a
Write the propagation steps leading to the formation of dichloromethane (CH2Cl2) from chloromethane.
Problem 2b
Explain why free-radical halogenation usually gives mixtures of products.
Problem 2c
How could an industrial plant control the proportions of methane and chlorine to favor production of CCl4? To favor production of CH3Cl?
Problem 3a
Each of the following proposed mechanisms for the free-radical chlorination of methane is wrong. Explain how the experimental evidence disproves each mechanism.
a.
Problem 3b
Each of the following proposed mechanisms for the free-radical chlorination of methane is wrong. Explain how the experimental evidence disproves each mechanism.
b.
- The bromination of methane proceeds through the following steps: 1. Br2 + 2 Br• ΔH° (per mole)/+190 kJ (45 kcal) Ea (per mole)/ 190 kJ (45 kcal) 2. CH4 + Br• —> CH3+ HBr +73 kJ (17 kcal) 79 kJ (19 kcal) 3. • CH3 + Br2 —> CH3Br + Br -112 kJ (-27 kcal) 4 kJ (1 kcal) a. Draw a complete reaction-energy diagram for this reaction. b. Label the rate-limiting step.
Problem 4
- The bromination of methane proceeds through the following steps: 1. Br2 + 2 Br• ΔH° (per mole)/+190 kJ (45 kcal) Ea (per mole)/ 190 kJ (45 kcal) 2. CH4 + Br• —> CH3+ HBr +73 kJ (17 kcal) 79 kJ (19 kcal) 3. • CH3 + Br2 —> CH3Br + Br -112 kJ (-27 kcal) 4 kJ (1 kcal) d. Compute the overall value of ΔH° for the bromination
Problem 4
- Use bond-dissociation enthalpies [TABLE 4-2], p. 167) to calculate values of ΔH° for the following reactions. a. CH3—CH3 + I2 —> CH3CH2I + HI
Problem 4
- Deuterium (D) is the hydrogen isotope of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C―D bond is slightly stronger than the C―H bond by 5.0 kJ/mol (1.2 kcal/ mol). Reaction rates tend to be slower when a C―D bond (as opposed to a C―H bond) is broken in a rate-limiting step. This effect, called a kinetic isotope effect, is clearly seen in the chlorination of methane. Methane undergoes free-radical chlorination 12 times as fast as tetradeuteriomethane (CD4) Faster: CH4 + Cl⋅ —> CH3Cl + HCl relative rate= 12 Slower: CD4 + Cl⋅ —> CD3Cl + DCl relative rate= 1 c. Consider the thermodynamics of the chlorination of methane and the chlorination of ethane, and use the Hammond postulate to explain why one of these reactions has a much larger isotope effect than the other.
Problem 4
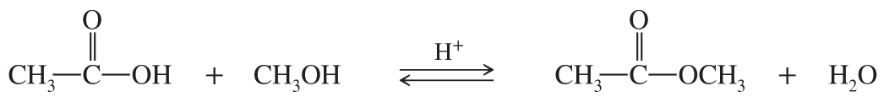
- The reaction of tert-butyl chloride with methanol (CH3)3C—Cl Tert-butylchloride + CH3—OH methanol —> (CH3)C—OCH3 methyltert-butylether + HCl is found to follow the rate equation rate= Kt[(CH3)3C—Cl] a. What is the kinetic order with respect to tert-butyl chloride?
Problem 4
- The reaction of tert-butyl chloride with methanol (CH3)3C—Cl Tert-butylchloride + CH3—OH methanol —> (CH3)C—OCH3 methyltert-butylether + HCl is found to follow the rate equation rate= Kr[(CH3)3C—Cl] c. What is the kinetic order overall?
Problem 4
Problem 4a,b
Free-radical chlorination of hexane gives very poor yields of 1-chlorohexane, while cyclohexane can be converted to chlorocyclohexane in good yield.
a. How do you account for this difference?
b. What ratio of reactants (cyclohexane and chlorine) would you use for the synthesis of chlorocyclohexane?
Problem 4.20
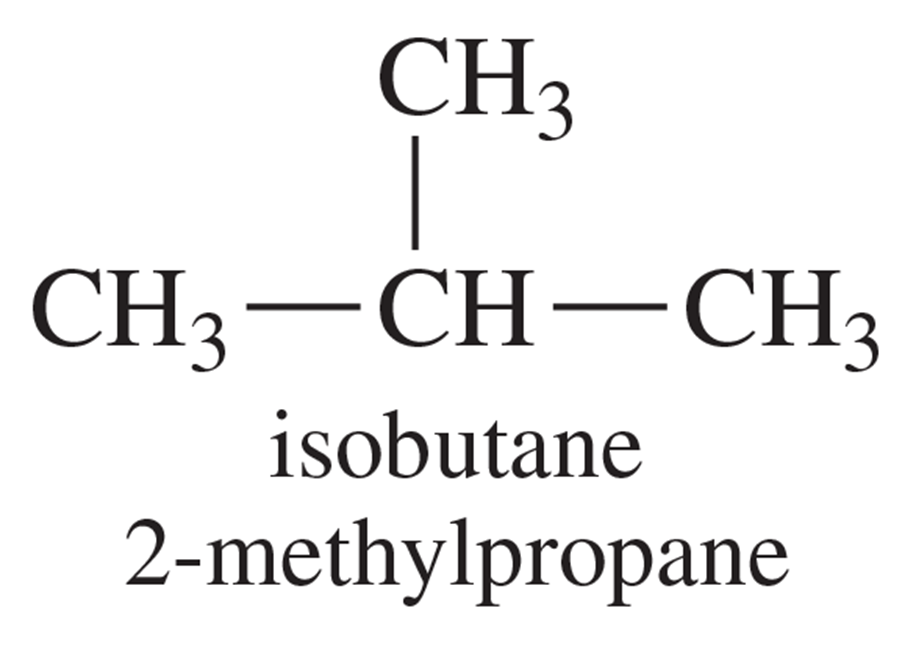
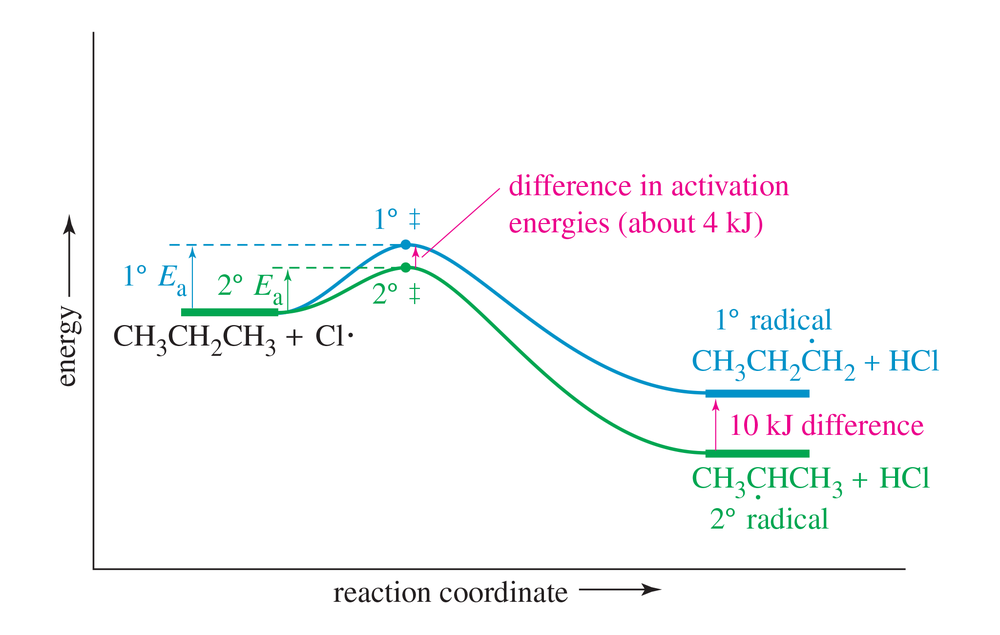
Use the bond-dissociation enthalpies in Table 4-2 (page 167) to calculate the heats of reaction for the two possible first propagation steps in the chlorination of isobutane. Use this information to draw a reaction-energy diagram like Figure 4-8, comparing the activation energies for formation of the two radicals.
Problem 5a
The following reaction has a value of ΔG° = –2.1 kJ/mol (–0.50 kcal/mol).
CH3Br + H2S ⇌ CH3SH + HBr
a. Calculate Keq at room temperature (25 °C) for this reaction as written.
Problem 5b
The following reaction has a value of ΔG° = –2.1 kJ/mol (–0.50 kcal/mol).
CH3Br + H2S ⇌ CH3SH + HBr
b. Starting with a 1 M solution of CH3Br and H2S, calculate the final concentrations of all four species at equilibrium.
Problem 6a
Under base-catalyzed conditions, two molecules of acetone can condense to form diacetone alcohol. At room temperature (25 °C), about 5% of the acetone is converted to diacetone alcohol. Determine the value of ΔG° for this reaction.
- The dehydrogenation of butane to trans-but-2-ene has ΔH° = +116 kJ/mol (+27.6 kcal/mol) and ΔS° = +117J/kelvin-mol (+28.0 cal/kelvin-mol). a. Compute the value of ΔG° for dehydrogenation at room temperature (25 °C or 298 °K). Is dehydrogenation favored or disfavored? HINT: When you are doing synthesis problems, avoid using these high-temperature industrial methods. They require specialized equipment, and they produce variable mixtures of products.
Problem 7
Problem 7a
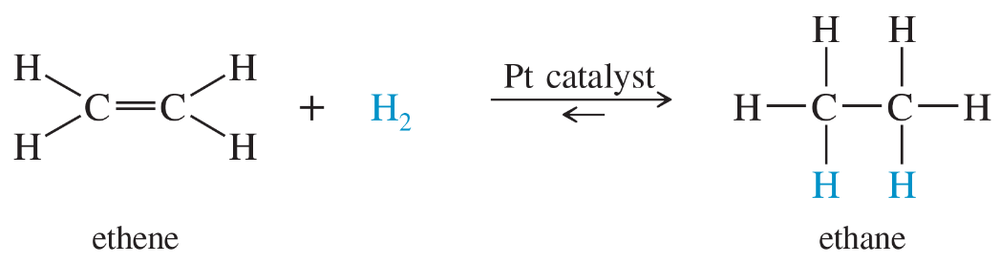
When ethene is mixed with hydrogen in the presence of a platinum catalyst, hydrogen adds across the double bond to form ethane. At room temperature, the reaction goes to completion. Predict the signs of ΔH° and ΔS° for this reaction. Explain these signs in terms of bonding and freedom of motion.
Problem 8a
For each reaction, estimate whether ΔS° for the reaction is positive, negative, or impossible to predict.
(a)
Problem 8b
For each reaction, estimate whether ΔS° for the reaction is positive, negative, or impossible to predict.
(b)
Problem 8c
For each reaction, estimate whether ΔS° for the reaction is positive, negative, or impossible to predict.
(c)
Problem 8.59
Draw a reaction-energy diagram for the propagation steps of the free-radical addition of HBr to isobutylene. Draw curves representing the reactions leading to both the Markovnikov and the anti-Markovnikov products. Compare the values of ∆Gº and Ea and for the rate-limiting steps, and explain why only one of these products is observed.
Problem 9a
a. Propose a mechanism for the free-radical chlorination of ethane,
Problem 9c,d
b. Calculate ΔH° for each step in this reaction.
c. Calculate the overall value of ΔH° for this reaction.
Problem 10a,b
a. Using bond-dissociation enthalpies from Table 4-2. (page 167), calculate the heat of reaction for each step in the free-radical bromination of methane
b. Calculate the overall heat of the reaction.
Problem 11a
The reaction of tert-butyl chloride with methanol
is found to follow the rate equation
rate = kr[(CH3)3C—Cl]
a. What is the kinetic order with respect to tert-butyl chloride?
Problem 11b
The reaction of tert-butyl chloride with methanol
is found to follow the rate equation
rate = kr[(CH3)3C—Cl]
b. What is the kinetic order with respect to methanol?
Problem 11c
The reaction of tert-butyl chloride with methanol
is found to follow the rate equation
rate = kr[(CH3)3C—Cl]
c. What is the kinetic order overall
Problem 12a
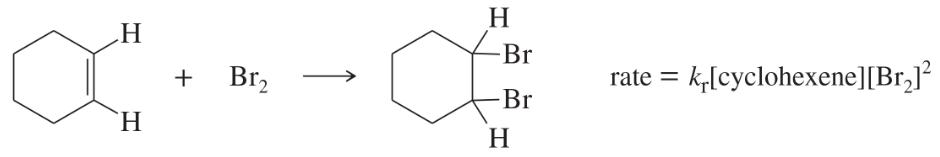
Under certain conditions, the bromination of cyclohexene follows an unusual rate law:
a. What is the kinetic order with respect to cyclohexene?