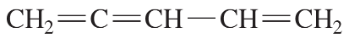Back
BackProblem 5a,b
Give the systematic (IUPAC) names of the following alkenes.
(a)
(b)
Problem 5d,e
Give the systematic (IUPAC) names of the following alkenes.
(d)
(e)
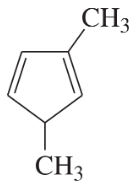
Problem 5f,g
Give the systematic (IUPAC) names of the following alkenes.
(f)
(g)
Problem 6a,b
1. Determine which of the following compounds show cis-trans isomerism.
2. Draw and name the cis and trans (or Z and E) isomers of those that do.
a. hex-3-ene
b.buta-1,3-diene
Problem 6c,d
1. Determine which of the following compounds show cis-trans isomerism.
2. Draw and name the cis and trans (or Z and E) isomers of those that do.
c. hexa-2,4-diene
d. 3-methylpent-2-ene
Problem 6e,f
1. Determine which of the following compounds show cis-trans isomerism.
2. Draw and name the cis and trans (or Z and E) isomers of those that do.
e. 2,3-dimethylpent-2-ene
f. 3,4-dibromocyclopentene
Problem 7a,b
The following names are all incorrect. Draw the structure represented by the incorrect name (or a consistent structure if the name is ambiguous), and give your drawing the correct name.
a. cis-dimethylpent-2-ene
b. 3-vinylhex-4-ene
Problem 7c,d
The following names are all incorrect. Draw the structure represented by the incorrect name (or a consistent structure if the name is ambiguous), and give your drawing the correct name.
c. 2-methylcyclopentene
d. 6-chlorocyclohexadiene
Problem 7e
The following names are all incorrect. Draw the structure represented by the incorrect name (or a consistent structure if the name is ambiguous), and give your drawing the correct name.
e. 2,5-dimethylcyclohexene
Problem 7-25b
For each reaction, decide whether substitution or elimination (or both) is possible, and predict the products you expect. Label the major products.
b. 1-bromo-1-methylcyclohexane + triethylamine(Et3N:)
Problem 8a,b
Some of the following examples can show geometric isomerism, and some cannot. For the ones that can, draw all the geometric isomers, and assign complete names using the E-Z system.
a. 3-bromo-2-chloropent-2-ene
b. 3-ethylhexa-2,4-diene
Problem 8c,d
Some of the following examples can show geometric isomerism, and some cannot. For the ones that can, draw all the geometric isomers, and assign complete names using the E-Z system.
c. 3-bromo-2-methylhex-3-ene
d. penta-1,3-diene
Problem 8e,f
Some of the following examples can show geometric isomerism, and some cannot. For the ones that can, draw all the geometric isomers, and assign complete names using the E-Z system.
e. 3-ethyl-5-methyloct-3-ene
f. 3,7-dichloroocta-2,5-diene
Problem 8g,h
Some of the following examples can show geometric isomerism, and some cannot. For the ones that can, draw all the geometric isomers, and assign complete names using the E-Z system.
(g)
(h)
Problem 8i
Some of the following examples can show geometric isomerism, and some cannot. For the ones that can, draw all the geometric isomers, and assign complete names using the E-Z system.
(i)
Problem 9a
How many stereogenic double bonds are in octa-1,3,5-triene? How many stereocenters are there? Draw and name the four stereoisomers of octa-1,3,5-triene.
Problem 9b
Draw the six stereoisomers of octa-2,4,6-triene. Explain why there are only six stereoisomers, rather than the eight we might expect for a compound with three stereogenic double bonds.
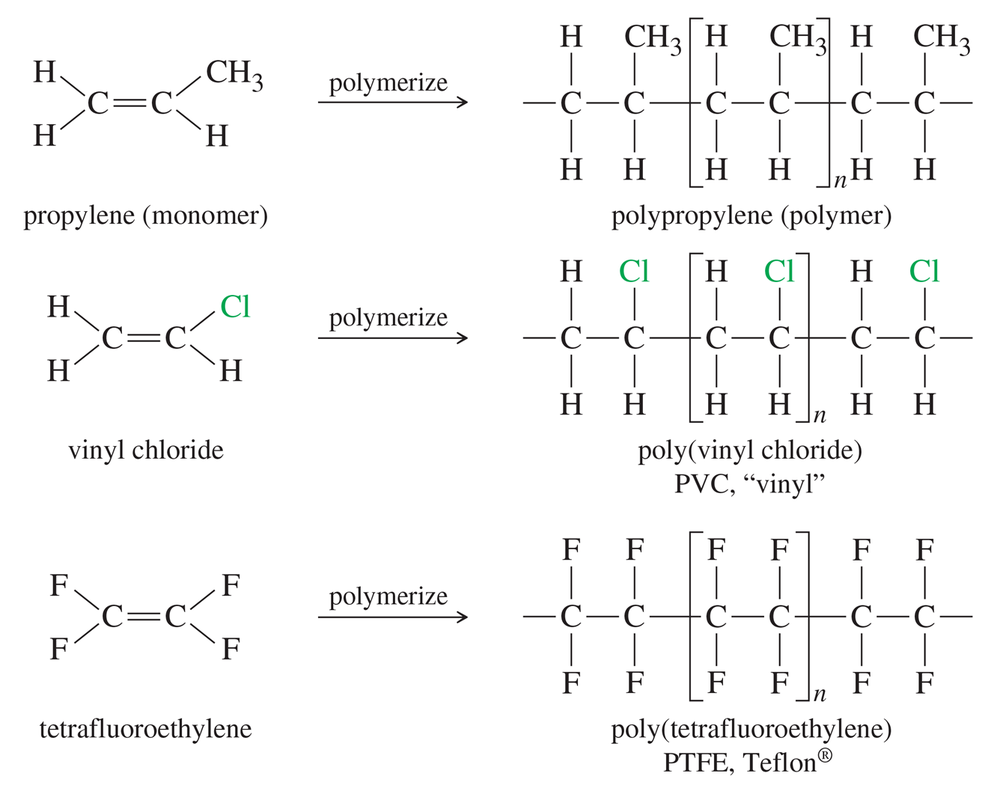
Problem 10a
Teflon-coated frying pans routinely endure temperatures that would cause polyethylene or polypropylene to oxidize and decompose. Decomposition of polyethylene is initiated by free-radical abstraction of a hydrogen atom by O2. Bond-dissociation energies of C—H bonds are about 400 kJ/mol, and C—F bonds are about 460 kJ/mol. The BDE of the H—OO bond is about 192 kJ/mol, and the F—OO bond is about 63 kJ/mol. Show why Teflon (Figure 7-5) is much more resistant to oxidation than polyethylene is.
Problem 11a
For each pair of compounds, predict the one with a higher boiling point. Which compounds have zero dipole moments?
a. cis-1,2-dichloroethene or cis-1,2-dibromoethene
Problem 11b
For each pair of compounds, predict the one with a higher boiling point. Which compounds have zero dipole moments?
b. cis- or trans-2,3-dichlorobut-2-ene
Problem 11c
For each pair of compounds, predict the one with a higher boiling point. Which compounds have zero dipole moments?
c. cyclohexene or 1,2-dichlorocyclohexene
Problem 12
Use the data in Table 7-2 to predict the energy difference between 2,3-dimethylbut-1-ene and 2,3-dimethylbut-2-ene. Which of these double-bond isomers is more stable?
Problem 13
Using Table 7-2 as a guide, predict which member of each pair is more stable, as well as by about how many kJ/mol or kcal/mol.
a. cis,cis-hexa-2,4-diene or trans,trans-hexa-2,4-diene
b. 2-methylbut-1-ene or 3-methylbut-1-ene
c. 2-methylbut-1-ene or 2-methylbut-2-ene
d. cis-4-methylpent-2-ene or 2-methylpent-2-ene
Problem 14a-d
Explain why each of the following alkenes is stable or unstable.
(a) 1,2-dimethylcyclopentene
(b) trans-1,2-dimethylcyclopentene
(c) trans-3,4-dimethylcyclopentene
(d) trans-1,2-dimethylcyclodecene
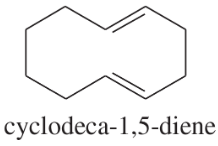
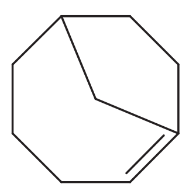
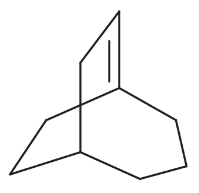
Problem 14h,i
Explain why each of the following alkenes is stable or unstable.
(h)
(i)
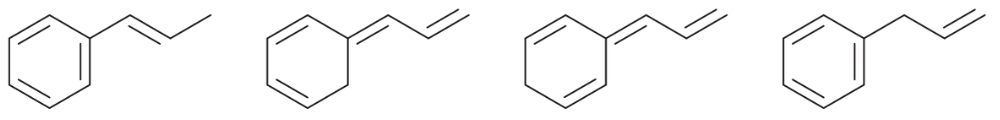
Problem 15a
For each set of isomers, choose the isomer that you expect to be most stable and the isomer you expect to be least stable.
(a)
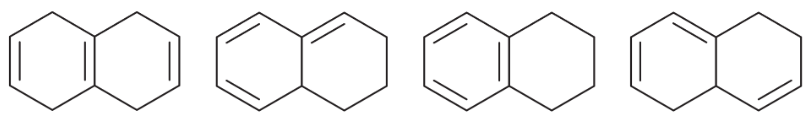
Problem 15b
For each set of isomers, choose the isomer that you expect to be most stable and the isomer you expect to be least stable.
(b)
Problem 15c
For each set of isomers, choose the isomer that you expect to be most stable and the isomer you expect to be least stable.
(c)
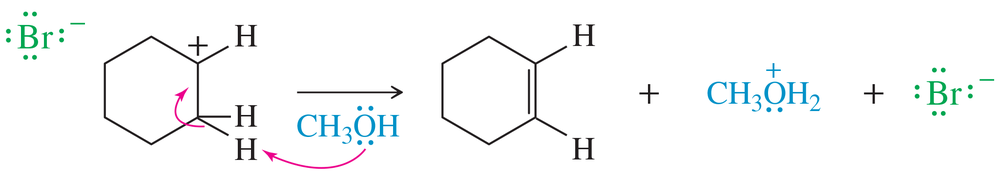
Problem 16
[FIGURE: KEY MECHANISM 7-1]
Show what happens in step 2 of the example if the solvent acts as a nucleophile (forming a bond to carbon) rather than as a base (removing a proton).
Problem 17a
SN1 substitution and E1 elimination frequently compete in the same reaction.
a. Propose a mechanism and predict the products for the solvolysis of 2-bromo-2,3,3-trimethylbutane in methanol.