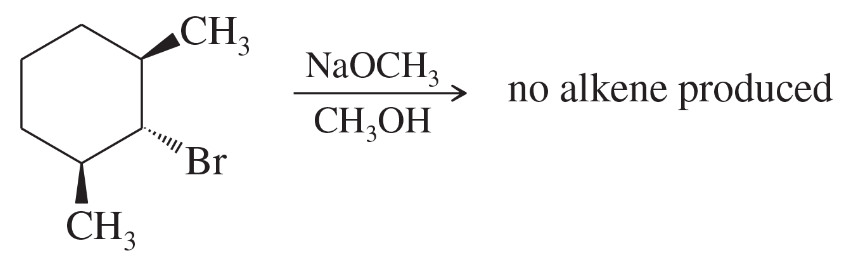Back
BackProblem 13
Using Table 7-2 as a guide, predict which member of each pair is more stable, as well as by about how many kJ/mol or kcal/mol.
a. cis,cis-hexa-2,4-diene or trans,trans-hexa-2,4-diene
b. 2-methylbut-1-ene or 3-methylbut-1-ene
c. 2-methylbut-1-ene or 2-methylbut-2-ene
d. cis-4-methylpent-2-ene or 2-methylpent-2-ene
Problem 14a-d
Explain why each of the following alkenes is stable or unstable.
(a) 1,2-dimethylcyclopentene
(b) trans-1,2-dimethylcyclopentene
(c) trans-3,4-dimethylcyclopentene
(d) trans-1,2-dimethylcyclodecene
Problem 14h,i
Explain why each of the following alkenes is stable or unstable.
(h)
(i)
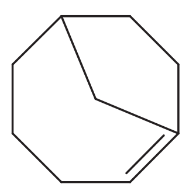
Problem 15a
For each set of isomers, choose the isomer that you expect to be most stable and the isomer you expect to be least stable.
(a)
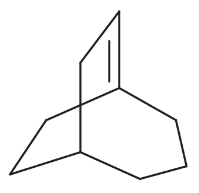
Problem 15b
For each set of isomers, choose the isomer that you expect to be most stable and the isomer you expect to be least stable.
(b)
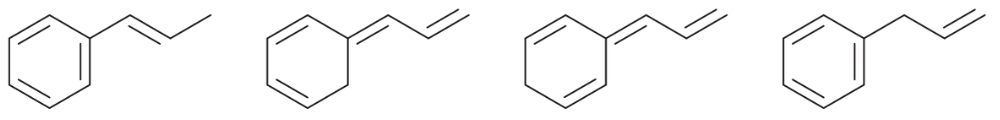
Problem 15c
For each set of isomers, choose the isomer that you expect to be most stable and the isomer you expect to be least stable.
(c)
Problem 16
[FIGURE: KEY MECHANISM 7-1]
Show what happens in step 2 of the example if the solvent acts as a nucleophile (forming a bond to carbon) rather than as a base (removing a proton).
Problem 17a
SN1 substitution and E1 elimination frequently compete in the same reaction.
a. Propose a mechanism and predict the products for the solvolysis of 2-bromo-2,3,3-trimethylbutane in methanol.
Problem 17b
SN1 substitution and E1 elimination frequently compete in the same reaction.
b. Compare the function of the solvent (methanol) in the E1 and SN1 reactions.
Problem 18a
Finish Solved Problem 7-3 by showing how the rearranged carbocations give the four products shown in the problem. Be careful when using curved arrows to show deprotonation and/or nucleophilic attack by the solvent. The curved arrows always show movement of electrons, not movement of protons or other species.
Problem 19
The solvolysis of 2-bromo-3-methylbutane potentially can give several products, including both E1 and products from both the unrearranged carbocation and the rearranged carbocation. Mechanisms 6-6 and 7-2 show the products from the rearranged carbocation. Summarize all the possible products, showing which carbocation they come from and whether they are the products of E1 or reactions.
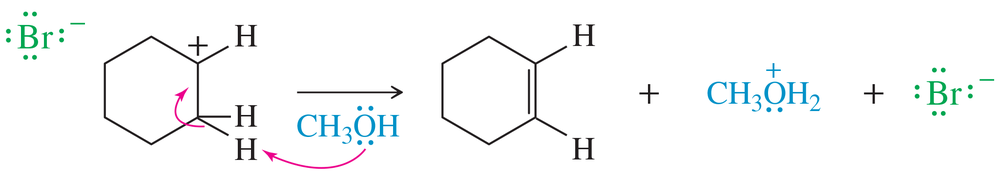
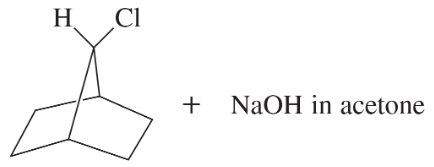
<IMAGES>
Problem 20a
Give the substitution and elimination products you would expect from the following reactions.
a. 3-bromo-3-ethylpentane heated in methanol
Problem 20b
Give the substitution and elimination products you would expect from the following reactions.
b. 1-iodo-1-phenylcyclopentane heated in ethanol
Problem 20c
Give the substitution and elimination products you would expect from the following reactions.
c. 1-bromo-2-methylcyclohexane + silver nitrate in water (AgNO3 forces ionization)
Problem 22a
When (1-bromoethyl)cyclohexane is heated in methanol for an extended period of time, five products result: two ethers and three alkenes. Predict the products of this reaction, and propose mechanisms for their formation.
Problem 22b
When (1-bromoethyl)cyclohexane is heated in methanol for an extended period of time, five products result: two ethers and three alkenes. Predict which of the three alkenes is the major elimination product.
Problem 23
Under second-order conditions (strong base/nucleophile), SN2 and E2 reactions may occur simultaneously and compete with each other. Show what products might be expected from the reaction of 2-bromo-3-methylbutane (a moderately hindered 2° alkyl halide) with sodium ethoxide.
Problem 24a
Predict the elimination products of the following reactions. When two alkenes are possible, predict which one will be the major product. Explain your answers, showing the degree of substitution of each double bond in the products.
a. 2-bromopentane + NaOCH3
b. 3-bromo-3-methylpentane + NaOMe (Me = methyl,CH3)
c. 2-bromo-3-ethylpentane + NaOH
Problem 24b
Which of these reactions are likely to produce both elimination and substitution products?
a. 2-bromopentane + NaOCH3
b. 3-bromo-3-methylpentane + NaOMe. (Me = methyl, CH3)
c. 2-bromo-3-ethylpentane + NaOH
Problem 25a
For each reaction, decide whether substitution or elimination (or both) is possible, and predict the products you expect. Label the major products.
a. 1−bromo−1−methylcyclohexane + NaOH in acetone
Problem 25c,d
For each reaction, decide whether substitution or elimination (or both) is possible, and predict the products you expect. Label the major products.
c. chlorocyclohexane+NaOCH3 in CH3OH
d. chlorocyclohexane + NaOC(CH3)3 in (CH3)3COH
Problem 26
Show that the (S,S) enantiomer of this (R,R) diastereomer of 1-bromo-1,2-diphenylpropane also undergoes E2 elimination to give the cis diastereomer of the product. (We do not expect these achiral reagents to distinguish between enantiomers.)
Problem 27a
Make models of the following compounds, and predict the products formed when they react with the strong bases shown.
(a)
Problem 27b
Make models of the following compounds, and predict the products formed when they react with the strong bases shown.
(b) meso-1,2-dibromo-1,2-diphenylethane + (CH3CH2)3N:
Problem 27c
Make models of the following compounds, and predict the products formed when they react with the strong bases shown.
(c) (d,l)-1,2-dibromo-1,2-diphenylethane + (CH3CH2)3N:
Problem 27d
Make models of the following compounds, and predict the products formed when they react with the strong bases shown.
(d)
Problem 28a
Predict the elimination products of the following reactions, and label the major products.
a. cis-1-bromo-2-methylcyclohexane + NaOCH3 in CH3OH
Problem 28b
Predict the elimination products of the following reactions, and label the major products.
b. trans-1-bromo-2-methylcyclohexane + NaOCH3 in CH3OH
Problem 29
When the following stereoisomer of 2-bromo-1,3-dimethylcyclohexane is treated with sodium methoxide, no E2 reaction is observed. Explain why this compound cannot undergo the E2 reaction in the chair conformation.
Problem 30a
Two stereoisomers of a bromodecalin are shown. Although the difference between these stereoisomers may seem trivial, one isomer undergoes elimination with KOH much faster than the other. Predict the products of these eliminations, and explain the large difference in the ease of elimination.