 Back
Back Bruice 8th Edition
Bruice 8th Edition Ch. 15 - Reactions of Carboxylic Acids and Carboxylic Acid Derivatives
Ch. 15 - Reactions of Carboxylic Acids and Carboxylic Acid DerivativesProblem 48d
Draw a structure for each of the following:
d. propanenitrile
Problem 48g
Draw a structure for each of the following:
g. benzoic anhydride
Problem 48i
Draw a structure for each of the following:
i. 3-methylbutanenitrile
Problem 48j
Draw a structure for each of the following:
j. cycloheptanecarboxylic acid
Problem 49a
Name the following:
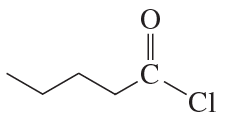
a.
Problem 49c
Name the following:
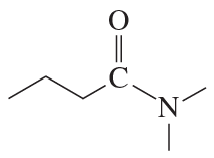
c.
Problem 49e
Name the following:
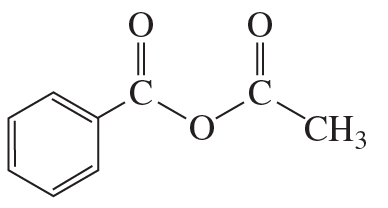
e.
Problem 49f
Name the following:
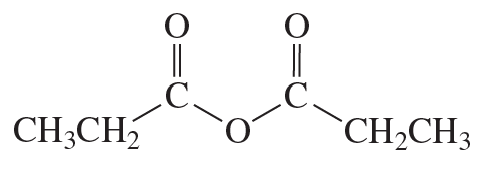
f.
Problem 50a,b
What compounds are formed from the reaction of benzoyl chloride with the following reagents?
a. sodium acetate
b. water
Problem 50e
What compounds are formed from the reaction of benzoyl chloride with the following reagents?
e. aqueous NaOH
Problem 50f
What compounds are formed from the reaction of benzoyl chloride with the following reagents?
f. cyclohexanol
Problem 50g,h
What compounds are formed from the reaction of benzoyl chloride with the following reagents?
g. excess benzylamine
h. 4-chlorophenol
Problem 50i
What compounds are formed from the reaction of benzoyl chloride with the following reagents?
i. isopropyl alcohol
Problem 50k
What compounds are formed from the reaction of benzoyl chloride with the following reagents?
k. potassium formate
Problem 51c
What compounds are obtained from the following hydrolysis reactions?
c.
Problem 51d
What compounds are obtained from the following hydrolysis reactions?
d.
Problem 53
Because bromocyclohexane is a secondary alkyl halide, both cyclohexanol and cyclohexene are formed when the alkyl halide reacts with hydroxide ion. Suggest a method to synthesize cyclohexanol from bromocyclohexane that forms little or no cyclohexene.
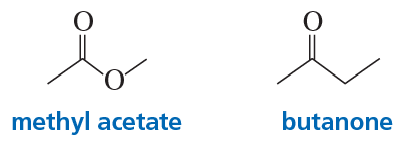
Problem 54
a. Which compound would you expect to have a greater dipole moment, methyl acetate or butanone?
b. Which would you expect to have a higher boiling point?
Problem 57d
Using an alcohol for one method and an alkyl halide for the other, show two ways to make each of the following esters:
d. methyl phenylethanoate (odor of honey)
Problem 58a,b
What reagents would you use to convert methyl propanoate to the following compounds?
a. isopropyl propanoate
b. sodium propanoate
Problem 58c,d
What reagents would you use to convert methyl propanoate to the following compounds?
c. N-ethylpropanamide
d. propanoic acid
Problem 59a
What products would you expect to obtain from the following reactions?
a. malonic acid + 2 acetyl chloride
Problem 59b
What products would you expect to obtain from the following reactions?
b. methyl carbamate + methylamine
Problem 59c,d
What products would you expect to obtain from the following reactions?
c. urea + water
d. β-ethylglutaric acid + acetyl chloride + Δ
Problem 60
A compound with molecular formula C5H10O2 gives the following IR spectrum. When it undergoes acid-catalyzed hydrolysis, the compound with the 1H NMR spectrum shown below is formed. Identify the compounds.
<IMAGE>
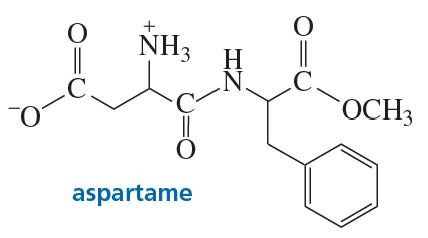
Problem 61
Aspartame, the sweetener used in the commercial products NutraSweet and Equal, is 200 times sweeter than sucrose. What products will be obtained if aspartame is hydrolyzed completely in an aqueous solution of HCl?
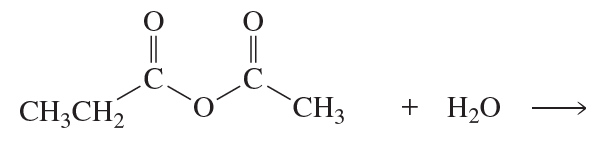
Problem 63(9,10)
a. Which of the following reactions does not give the carbonyl product shown?
b. Which of the reactions that do not occur can be made to occur if an acid catalyst is added to the reaction mixture?
9.
10.
Problem 63(5)
a. Which of the following reactions does not give the carbonyl product shown?
b. Which of the reactions that do not occur can be made to occur if an acid catalyst is added to the reaction mixture?
5.
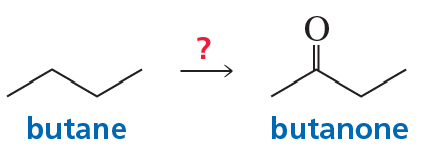
Problem 64
Describe how the target molecule (butanone) can be synthesized in a high yield from butane.
Problem 65
1,4-Diazabicyclo[2.2.2]octane (abbreviated DABCO) is a tertiary amine that catalyzes transesterification reactions. Explain how it does this.
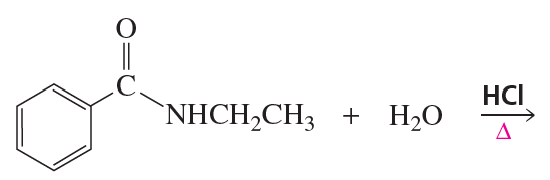
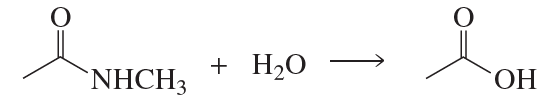
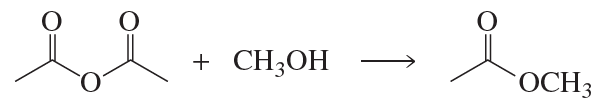
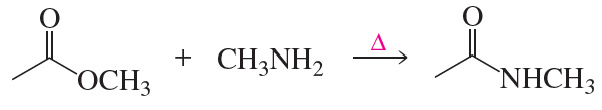
![Chemical structure of 1,4-Diazabicyclo[2.2.2]octane (DABCO), a catalyst for transesterification reactions.](https://static.studychannel.pearsonprd.tech/courses/organic-chemistry/thumbnails/c7c3cb11-70de-4a8d-9feb-cf5d5fcb7ded)