Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.3 - An Introduction to Organic Compounds:Nomenclature, Physical Properties, and Structure
Ch.3 - An Introduction to Organic Compounds:Nomenclature, Physical Properties, and StructureProblem 45
Draw 1,2,3,4,5,6-hexachlorocyclohexane with
a. all the chloro groups in axial positions.
b. all the chloro groups in equatorial positions.
Problem 46
Using the data in Table 3.9, calculate the percentage of molecules of cyclohexanol that have the OH group in an equatorial position at 25 °C.
Problem 47
The chair conformer of fluorocyclohexane is 0.25 kcal/mol more stable when the fluoro substituent is in an equatorial position than when it is in an axial position. How much more stable is the anti conformer than a gauche conformer of 1-fluoropropane, considering rotation about the C1−C2 bond?
Problem 48a,b,c
Is each of the following a cis isomer or a trans isomer?
a,
b.
c.
Problem 48d,e,f
Is each of the following a cis isomer or a trans isomer?
d.
e.
f.
Problem 49
Which has a higher percentage of the diequatorial-substituted conformer compared with the diaxialsubstituted conformer: trans-1,4-dimethylcyclohexane or cis-1-tert-butyl-3-methylcyclohexane?
Problem 50a
Draw the more stable chair conformer of cis-1-ethyl-2-methylcyclohexane.
Problem 50c
Which compound is more stable: cis-1-ethyl-2-methylcyclohexane or trans-1-ethyl-2-methylcyclohexane?
Problem 51a,b
For each of the following disubstituted cyclohexanes, indicate whether the substituents in the two chair conformers are both equatorial in one chair conformer and both axial in the other or one equatorial and one axial in each of the chair conformers:
a. cis-1,2-
b. trans-1,2-
Problem 51c,d
For each of the following disubstituted cyclohexanes, indicate whether the substituents in the two chair conformers are both equatorial in one chair conformer and both axial in the other or one equatorial and one axial in each of the chair conformers:
c. cis-1,3-
d. trans-1,3-
Problem 51e,f
For each of the following disubstituted cyclohexanes, indicate whether the substituents in the two chair conformers are both equatorial in one chair conformer and both axial in the other or one equatorial and one axial in each of the chair conformers:
e. cis-1,4-
f. trans-1,4-
Problem 53
a. Draw Newman projections of the two conformers of trans-1,3-dimethylcyclohexane.
b. Which of the conformers predominates at equilibrium?
Problem 54a
Calculate the energy difference between the two chair conformers of trans-1,4-dimethylcyclohexane.
Problem 54b
What is the energy difference between the two chair conformers of cis-1,4-dimethylcyclohexane?
Problem 55a
Draw a condensed structure and a skeletal structure for each of the following:
a. sec-butyl tert-butyl ether
Problem 55a,b
Draw a condensed structure and a skeletal structure for each of the following:
a. sec-butyl tert-butyl ether
b. isoheptyl alcohol
Problem 55c,d
Draw a condensed structure and a skeletal structure for each of the following:
c. sec-butylamine
d. isopentyl bromide
Problem 55g
Draw a condensed structure and a skeletal structure for each of the following:
g. 4-(1,1-dimethylethyl)heptane
Problem 55h
Draw a condensed structure and a skeletal structure for each of the following:
h. 5,5-dibromo-2-methyloctane
Problem 55i
Draw a condensed structure and a skeletal structure for each of the following:
i. 3-ethoxy-2-methylhexane
Problem 55k
Draw a condensed structure and a skeletal structure for each of the following:
k. 3,4-dimethyloctane
Problem 55l
Draw a condensed structure and a skeletal structure for each of the following:
l. 5-isopropyldecane
Problem 57(3,4)
a. What is each compound’s systematic name?
b. Draw a skeletal structure for each condensed structure given and draw a condensed structure for each skeletal structure.
3.
4. (CH3CH2)4C
Problem 57(7,8)
a. What is each compound’s systematic name?
b. Draw a skeletal structure for each condensed structure given and draw a condensed structure for each skeletal structure.
7.
8. CH3OCH2CH2CH2OCH3
Problem 57(5,6)
a. What is each compound’s systematic name?
b. Draw a skeletal structure for each condensed structure given and draw a condensed structure for each skeletal structure.
5. BrCH2CH2CH2CH2CH2NHCH2CH3
6.
Problem 57(1,2)
a. What is each compound’s systematic name?
b. Draw a skeletal structure for each condensed structure given and draw a condensed structure for each skeletal structure.
1. (CH3)3CCH2CH2CH2CH(CH3)2
2.
Problem 58
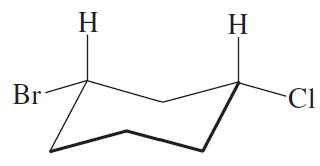
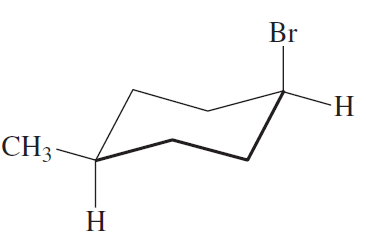
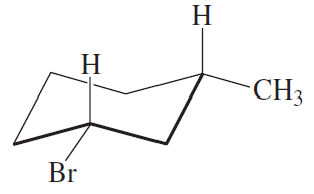
Which of the following represents a cis isomer?
Problem 59(1)
a. How many primary carbons does each of the following compounds have?
b. How many secondary carbons does each one have?
c. How many tertiary carbons does each one have?
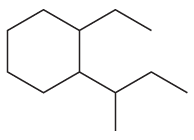
1.
Problem 59(2)
a. How many primary carbons does each of the following compounds have?
b. How many secondary carbons does each one have?
c. How many tertiary carbons does each one have?
2.
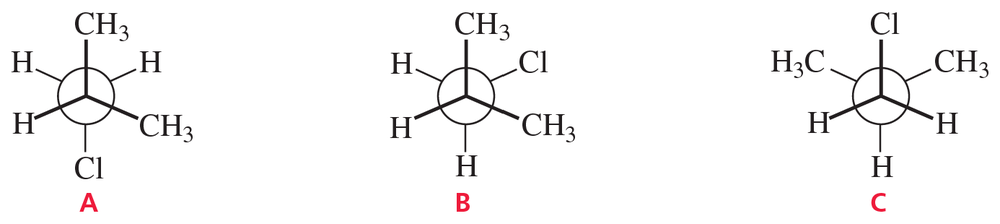
Problem 60
Which of the following conformers of isobutyl chloride is the most stable?