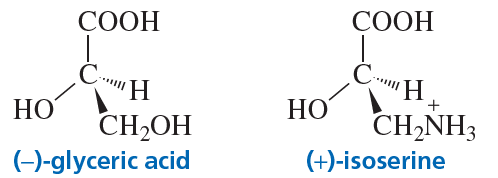Back
BackProblem 25
Draw a perspective formula for each of the following:
a. (S)-2-chlorobutane
b. (R)-1,2-dibromobutane
Problem 27
Convert the Fischer projection to a perspective formula.
Problem 28a
a. Is (R)-lactic acid dextrorotatory or levorotatory?
b. Is (R)-sodium lactate dextrorotatory or levorotatory?
Problem 30a,b
What is the configuration of the following compounds? (Use the given structures to answer the question.)
a. ()-glyceric acid
b. ()-isoserine
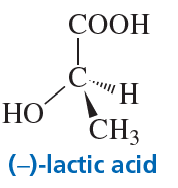
Problem 30c
What is the configuration of the following compounds? (Use the given structures to answer the question.)
c. ()-lactic acid
Problem 32
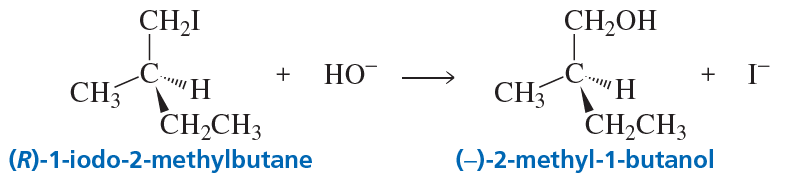
The reaction of (R)-1-iodo-2-methylbutane with hydroxide ion forms an alcohol without breaking any bonds to the asymmetric center. The alcohol rotates the plane of polarization of plane-polarized light counterclockwise. What is the configuration of (+)-2-methyl-1-butanol?
Problem 33
The observed rotation of 2.0 g of a compound in 50 mL of solution in a polarimeter tube 20 cm long is +138°. What is the specific rotation of the compound?
Problem 35c
(+)-Mandelic acid has a specific rotation of +158. What would be the observed specific rotation of each of the following mixtures?
c. 75% (-)-mandelic acid and 25% (+)-mandelic acid
Problem 36
Naproxen, a nonsteroidal anti-inflammatory drug that is the active ingredient in Aleve (p. 115), has a specific rotation of +66. One commercial preparation results in a mixture with a 97% enantiomeric excess.
a. Does naproxen have the R or the S configuration?
b. What percent of each enantiomer is obtained from the commercial preparation?
Problem 38
The following compound has only one asymmetric center. Why then does it have four stereoisomers?
Problem 39
a. Stereoisomers with two asymmetric centers are called ___ if the configuration of both asymmetric centers in one stereoisomer is the opposite of the configuration of the symmetric centers in the other stereoisomer.
b. Stereoisomers with two asymmetric centers are called ___ if the configuration of both asymmetric centers in one stereoisomer is the same as the configuration of the asymmetric centers in the other stereoisomer.
c. Stereoisomers with two asymmetric centers are called ___ if one of the asymmetric centers has the same configuration in both stereoisomers and the other asymmetric center has the opposite configuration in the two stereoisomers.
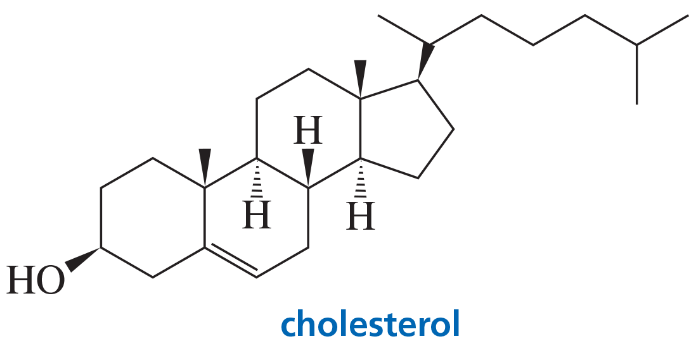
Problem 40
The stereoisomer of cholesterol found in nature is shown here.
a. How many asymmetric centers does cholesterol have?
b. What is the maximum number of stereoisomers that cholesterol can have?
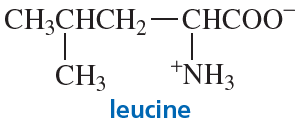
Problem 41a
Draw the stereoisomers of the following amino acids. Indicate pairs of enantiomers and pairs of diastereomers.
a.
Problem 42
1-Bromo-2-methylcyclopentane has four pairs of diastereomers. Draw the four pairs.
Problem 44b
Draw all possible stereoisomers for each of the following:
b. 2-bromo-4-chlorohexane
Problem 45
Draw the stereoisomers of 2-methylcyclohexanol.
Problem 46
Of all the possible cyclooctanes that have one chloro substituent and one methyl substituent, which ones do not have any asymmetric centers?
Problem 47c,d
Draw a diastereomer for each of the following:
c.
d.
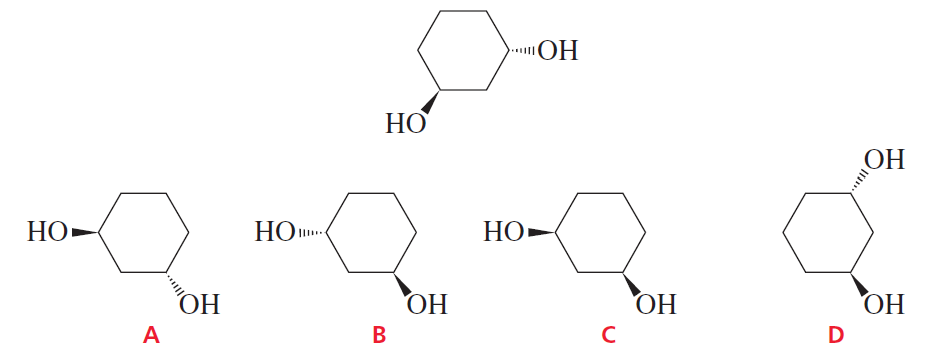
Problem 48
Indicate whether each of the structures in the second row is an enantiomer of, is a diastereomer of, or is identical to the structure in the top row.
Problem 49a,b
Which of the following compounds has a stereoisomer that is a meso compound?
a. 2,4-dibromohexane
b. 2,4-dibromopentane
Problem 49c,d
Which of the following compounds has a stereoisomer that is a meso compound?
c. 2,4-dimethylpentane
d. 1,3-dichlorocyclohexane
Problem 49e,f
Which of the following compounds has a stereoisomer that is a meso compound?
e. 1,4-dichlorocyclohexane
f. 1,2-dichlorocyclobutane
Problem 51e,f
Draw all the stereoisomers for each of the following:
e. 3,4-dichlorohexane
f. 1,2-dichlorocyclobutane
Problem 51g,h
Draw all the stereoisomers for each of the following:
g. 1,3-dichlorocyclohexane
h. 1,4-dichlorocyclohexane
Problem 52a
Draw the four stereoisomers of 1,3-dichloro-2-pentanol using
a. Fischer projections.
Problem 52b
Draw the four stereoisomers of 1,3-dichloro-2-pentanol using
b. perspective formulas.
Problem 53
Name the isomers you drew in Problem 52.
Problem 54
Chloramphenicol is a broad-spectrum antibiotic that is particularly useful against typhoid fever. What is the configuration of each of its asymmetric centers?
Problem 55a,b
Draw a perspective formula for each of the following:
a. (S)-3-chloro-1-pentanol
b. (2R,3R)-2,3-dibromopentane
Problem 55c,d
Draw a perspective formula for each of the following:
c. (2S,3R)-3-methyl-2-pentanol
d. (R)-1,2-dibromobutane