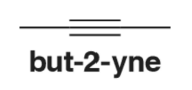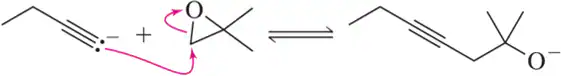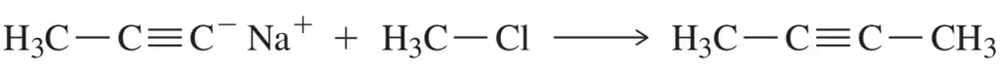Back
BackProblem 2
Calculate the oxidation numbers for each indicated atom in norethindrone, a steroid contraceptive.
Problem 3
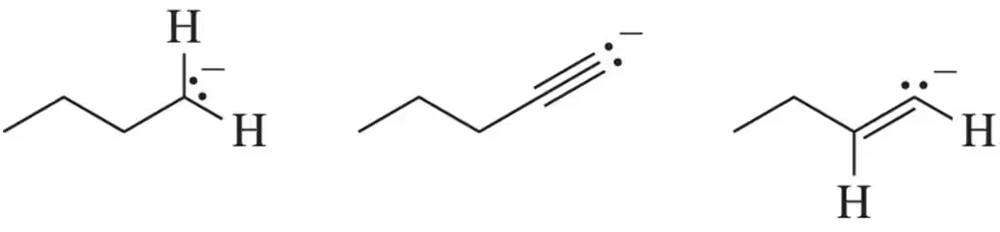
Rank the reactivity of the following anions with a general electrophile from least to most reactive.
Problem 4
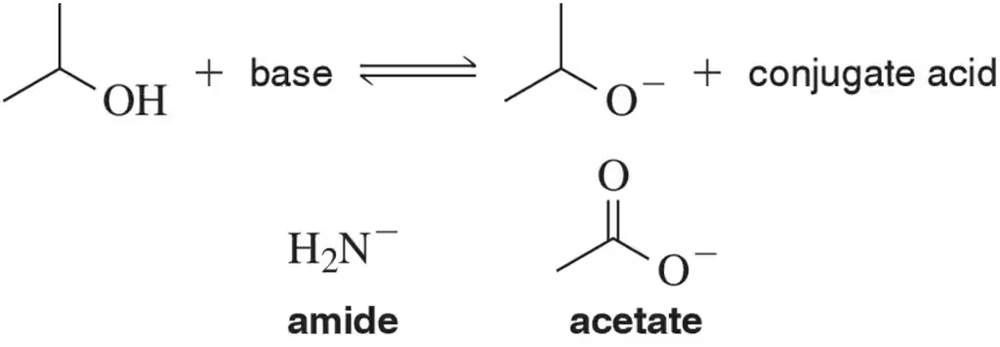
Would amide or acetate most easily deprotonate an alcohol to make the alkoxide anion? Calculate Keq for each possibility.
Problem 5
The following reaction gives two different constitutional isomers in nearly equal yields. Why doesn't this reaction produce only one product?
Problem 6a
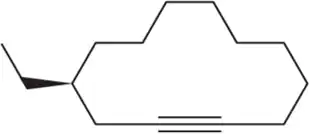
Identify the following alkynes as terminal (T), internal/symmetrical (IS), or internal/unsymmetrical (IU).
(a)
Problem 6c
Identify the following alkynes as terminal (T), internal/symmetrical (IS), or internal/unsymmetrical (IU).
(c)
Problem 6f
Identify the following alkynes as terminal (T), internal/symmetrical (IS), or internal/unsymmetrical (IU).
(f)
Problem 7
Draw all internal symmetrical alkynes with the molecular formula C10H18.
Problem 7.19
The following reaction was recently reported to have been performed electrochemically.
(a) Identify the reagent and a solvent that could have been used if this reaction was done traditionally.
(b) What safety and environmental hazards are improved or worsened when doing this reaction electrochemically?
Problem 8b
Provide the IUPAC names for the following alkynes.
(b)
Problem 9a
Draw the structures that correspond to the following IUPAC names.
(a) (R)-4-isopropyl-6-methylhept-2-yne
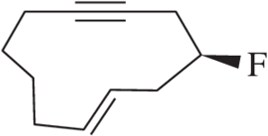
Problem 9c
Draw the structures that correspond to the following IUPAC names.
(c) (S)-3-fluoropent-1-yne
Problem 10c
Provide the IUPAC name for the following molecules.
(c)
Problem 11b
Draw the structures that correspond to the following names.
(b) (3Z,8S)-8-ethyl-3-methylundec-3-en-6-yne
Problem 11c
Draw the structures that correspond to the following names.
(c) (Z)-2-chloro-7-methyloct-2-en-4-yne
Problem 12
Draw the molecular orbital picture of but-2-yne.
Problem 13
Which of the following would be expected to give a hotter flame during combustion? Explain.
Problem 15a
Calculate Keq for the following acid–base reactions. Which is the best base to use to deprotonate acetylene and make the acetylide anion?
(a) HO- + H―C ≡ C―H ⇌ -C ≡ C―H + H2O
Problem 15b
Calculate for the following acid–base reactions. Which is the best base to use to deprotonate acetylene and make the acetylide anion?
(b) H2N- + H―C ≡ C―H ⇌ -C ≡ C―H + H3N
Problem 15c
Calculate for the following acid–base reactions. Which is the best base to use to deprotonate acetylene and make the acetylide anion?
(c)
Problem 16
Using pKa values, calculate an approximate Keq value for the following substitution reaction.
Problem 17a
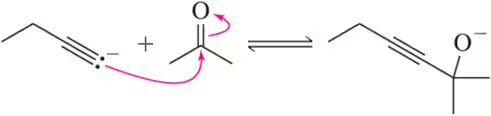
Estimate the Keq for the following reactions based on the stability of the anions involved.
(a)
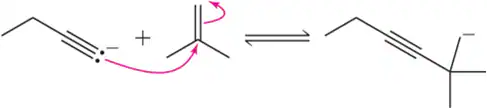
Problem 17b
Estimate the Keq for the following reactions based on the stability of the anions involved.
(b)
Problem 17c
Estimate the Keq for the following reactions based on the stability of the anions involved.
(c)
Problem 18
Draw a transition state for the following substitution reaction.
Problem 19a
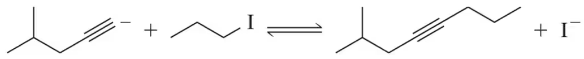
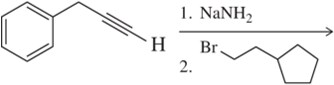
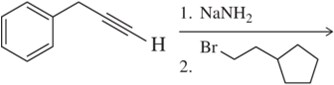
Predict the product of the following acetylide alkylations.
(a)
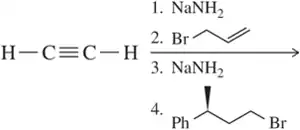
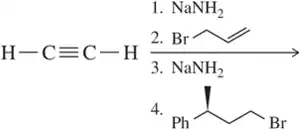
Problem 19c
Predict the product of the following acetylide alkylations.
(c)
Problem 20a
Suggest reagents you might use to generate the product from the given reactant.
(a)
Problem 20b
Suggest reagents you might use to generate the product from the given reactant.
(b)
Problem 20c
Suggest reagents you might use to generate the product from the given reactant.
(c)