Back
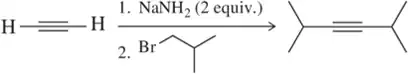
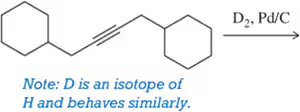
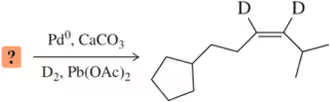
BackProblem 21a
A student provided the product of the following reactions, but made a mistake. Identify the mistake, correct the mistake, and suggest a way for the student to avoid that mistake in the future.
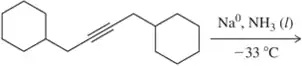
(a)
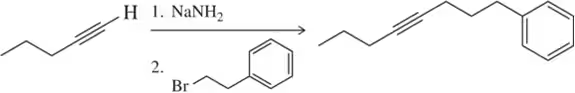
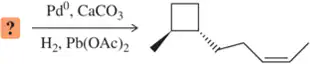
Problem 21b
A student provided the product of the following reactions, but made a mistake. Identify the mistake, correct the mistake, and suggest a way for the student to avoid that mistake in the future.
(b)
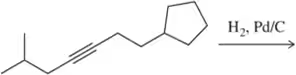
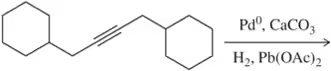
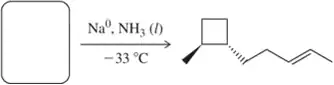
Problem 22a
Predict the product of the following hydrogenation reactions.
(a)
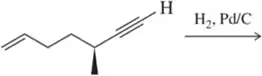
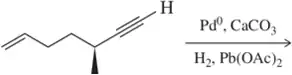
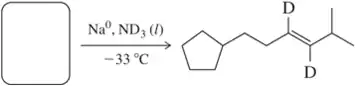
Problem 22b
Predict the product of the following hydrogenation reactions.
(b)
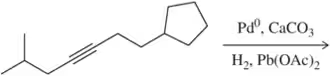
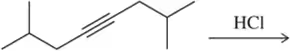
Problem 22c
Predict the product of the following hydrogenation reactions.
(c)
Problem 23a
Predict the product of the following hydrogenation reactions run with a poisoned catalyst.
(a)
Problem 23b
Predict the product of the following hydrogenation reactions run with a poisoned catalyst.
(b)
Problem 23c
Predict the product of the following hydrogenation reactions run with a poisoned catalyst.
(c)
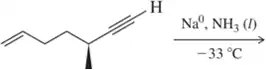
Problem 24b
Hydrogenation of which alkynes would produce the following cis-alkenes?
(b)
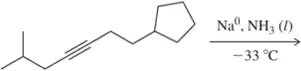
Problem 24c
Hydrogenation of which alkynes would produce the following cis-alkenes?
(c)
Problem 25a
Predict the product of the following alkyne reductions.
(a)
Problem 25b
Predict the product of the following alkyne reductions.
(b)
Problem 25c
Predict the product of the following alkyne reductions.
(c)
Problem 26a
Suggest alkynes that might be used to make the following trans-alkenes.
(a)
Problem 26b
Suggest alkynes that might be used to make the following trans-alkenes.
(b)
Problem 26c
Suggest alkynes that might be used to make the following trans-alkenes.
(c)
Problem 27a
The following deprotonation step occurs during the trans reduction of an alkyne. Calculate Keq for this reaction.
Problem 28
When sodium generates electrons in the presence of ammonia, these electrons persist in solution, giving the blue color. However, electrons do not persist when sodium is added to water. Why?
Problem 29
Sodium amide, the base we use to deprotonate terminal alkynes, is synthesized by reducing ammonia via a mechanism similar to the reduction of alkynes in Figure 10.21. Suggest a mechanism for this reaction.
Problem 30
Long, polarized bonds are also reducible in the same way that the C―C π bond of an alkyne is. Show a mechanism by which a C―Br bond might be reduced by sodium metal.
Problem 31b
We have studied the following reactions in previous chapters. For each, (i) indicate which reaction sheets they should appear on, (ii) show the best structure to use to represent them, and (iii) write the notes you could put in the margin so that the mechanism is implied.
(b) Radical halogenation of an alkane
Problem 32
The alkyl carbocation is estimated to be 15 kcal/mol more stable than the alkenyl carbocation. If this is also the difference in the energies of the transition state leading to each, what is the expected rate difference?
<IMAGE>
Problem 33b
Predict the major products resulting from the addition of one equivalent of HX to the following alkynes.
(b)
Problem 34a
Predict the alkyne and reactants you might use to make the following haloalkenes. [Providing the reactant and the reagent is how we start thinking about synthesis.]
(a)
Problem 34b
Predict the alkyne and reactants you might use to make the following haloalkenes. [Providing the reactant and the reagent is how we start thinking about synthesis.]
(b)
Problem 37
Draw the ketone(s) you would expect to form by reacting the following alkynes under the conditions of oxymercuration: (a) 6-methyloct-1-yne, (b) 1,10-dicyclohexyldec-5-yne, and (c) 5-phenylhex-2-yne.
Problem 38
Draw the ketone(s) you would expect to form by treating the following alkynes under the conditions of hydroboration–oxidation: (a) 6-methyloct-1-yne, (b) 1,10-dicyclohexyldec-5-yne, and (c) 5-phenylhex-2-yne.
<IMAGE>
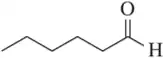
Problem 39a
For each of the following ketones/aldehydes, indicate whether it is possible to synthesize it from an alkyne as the only compound in good ( > 50%) yield. If so, how would you do it?
(a)
Problem 39d
For each of the following ketones/aldehydes, indicate whether it is possible to synthesize it from an alkyne as the only compound in good (> 50%) yield. If so, how would you do it?
(d)
Problem 40b
When doing synthesis, you will often find yourself repeating the same series of steps. To see this in action, synthesize the following aldehydes beginning with an organic molecule containing three carbons or fewer.
(b)