Back
BackProblem 40d
When doing synthesis, you will often find yourself repeating the same series of steps. To see this in action, synthesize the following aldehydes beginning with an organic molecule containing three carbons or fewer.
(d)
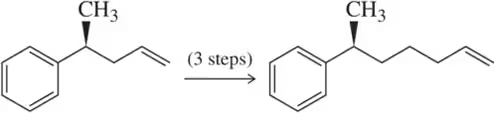
Problem 41a
Beginning with the molecules on the left of each chemical equation, synthesize the molecules shown. While there can be multiple ways of doing each synthesis, the minimum number of steps necessary is indicated over each reaction arrow.
(a)
Problem 41b
Beginning with the molecules on the left of each chemical equation, synthesize the molecules shown. While there can be multiple ways of doing each synthesis, the minimum number of steps necessary is indicated over each reaction arrow.
(b)
Problem 41c
Beginning with the molecules on the left of each chemical equation, synthesize the molecules shown. While there can be multiple ways of doing each synthesis, the minimum number of steps necessary is indicated over each reaction arrow.
(c)
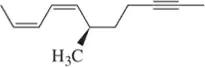
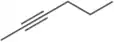
Problem 42b
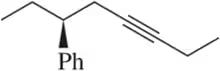
Name the following alkynes according to the IUPAC rules of nomenclature.
(b)
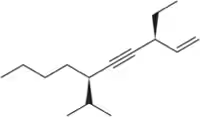
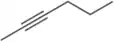
Problem 42c
Name the following alkynes according to the IUPAC rules of nomenclature.
(c)
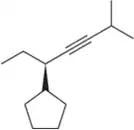
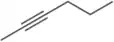
Problem 42d
Name the following alkynes according to the IUPAC rules of nomenclature.
(d)
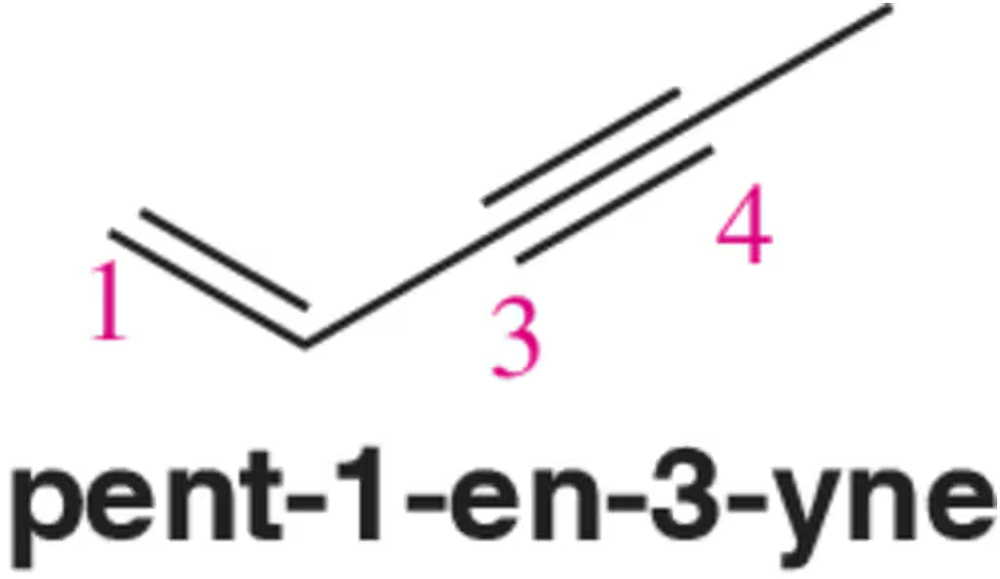
Problem 43a
Draw the molecular orbital picture of pent-1-en-3-yne.
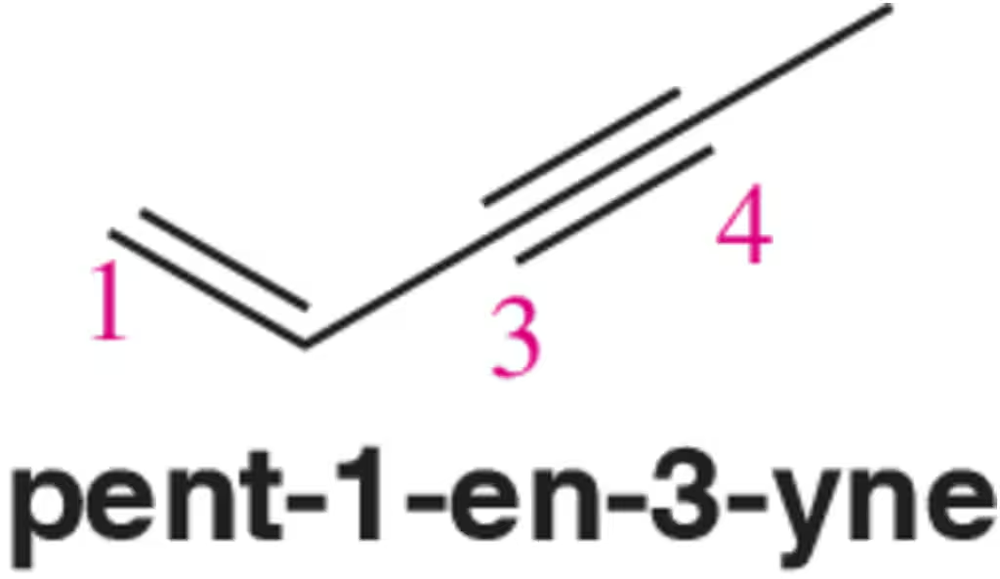
Problem 43b
To which carbon would you expect an electrophile to add (1, 3, or 4)? Explain your answer.
Problem 44c(i)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (i) H2 , Pd/C; If you expect two products, show both.
(c)
Problem 44c(ii)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (ii) H2, Pd/C, Pb(OAc)2 , CaCO3 (Lindlar's catalyst). If you expect two products, show both.
(c)
Problem 44c(iii)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (iii) Na0 , NH3 (liquid), ―33°C . If you expect two products, show both.
(c)
Problem 44c(iv)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (iv) HBr (1 equiv.). If you expect two products, show both.
(c)
Problem 44c(v)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (v) HCl (2 equiv.). If you expect two products, show both.
(c)
Problem 44c(vi)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (vi) H2SO4, HgSO4, H2O. If you expect two products, show both.
(c)
Problem 44c(vii)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (vii) 1. BH3 2. H2O2, NaOH. If you expect two products, show both.
(c)
Problem 44c(viii)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (viii) Cl2 (1 equiv.). If you expect two products, show both.
(c)
Problem 44c(ix)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (ix) Br2 (2 equiv.). If you expect two products, show both.
(c)
Problem 44f(ix)
For the alkynes shows here, show the product(s) expected to form when treated under the following conditions: (ix) Br2 (2 equiv.). If you expect two products, show both.
(f)
Problem 45a
Show the products of the following acetylide alkylation reactions. [Make sure your product has the correct number of carbons.]
(a)
Problem 45b
Show the products of the following acetylide alkylation reactions. [Make sure your product has the correct number of carbons.]
(b)
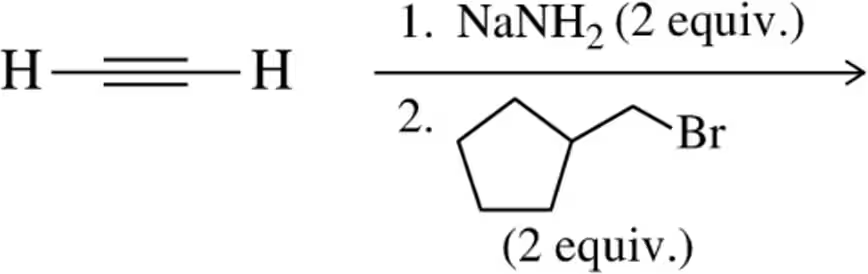
Problem 45c
Show the products of the following acetylide alkylation reactions. [Make sure your product has the correct number of carbons.]
(c)
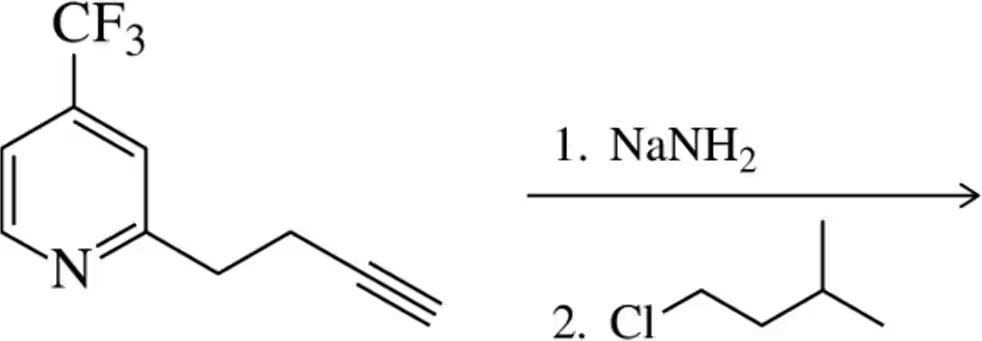
Problem 45d
Show the products of the following acetylide alkylation reactions. [Make sure your product has the correct number of carbons.]
(d)
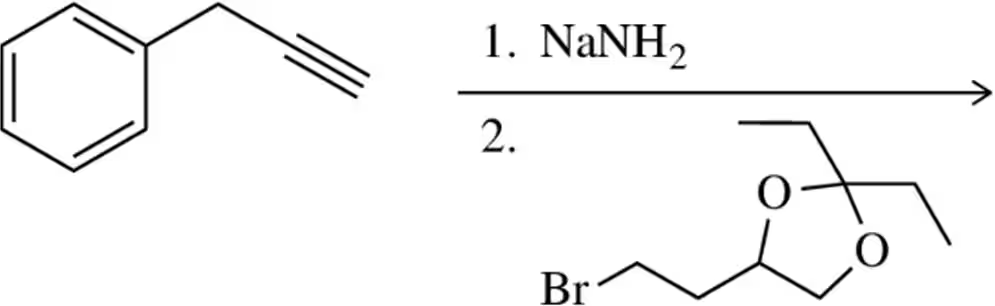
Problem 46
Complete the following synthesis by providing the necessary reagents.
Problem 47a
Suggest a method for synthesizing the following alkynes using an alkyne and an alkyl halide. [There are two correct answers for each product.]
(a)
Problem 47c
Suggest a method for synthesizing the following alkynes using an alkyne and an alkyl halide. [There are two correct answers for each product.]
(c)
Problem 48a
Beginning with the molecules on the left, provide a synthesis of the molecule on the right. The ideal number of steps is indicated over the reaction arrow, although there may be alternate routes worth considering.
(a)
Problem 48b
Beginning with the molecules on the left, provide a synthesis of the molecule on the right. The ideal number of steps is indicated over the reaction arrow, although there may be alternate routes worth considering.
(b)
Problem 49b
Synthesize the following molecules beginning with only organic molecules containing three carbons or fewer.
(b)
Problem 49e
Synthesize the following molecules beginning with only organic molecules containing three carbons or fewer.
(e)