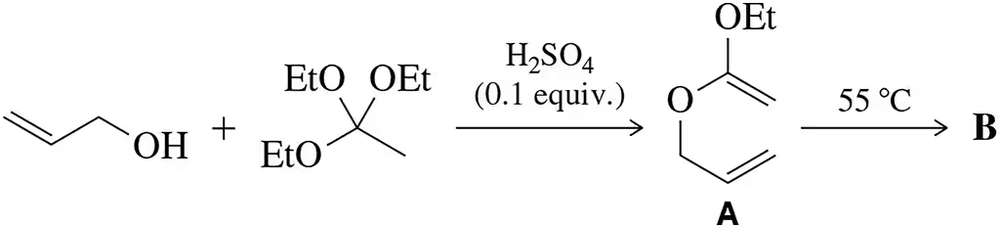Back
BackProblem 35
Provide a mechanism of the Diels–Alder reaction shown and predict the regioisomer that will form.
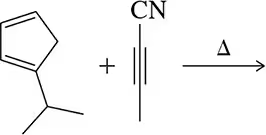
Problem 36a
Predict the product of the Diels–Alder reactions shown.
(a)
Problem 37a
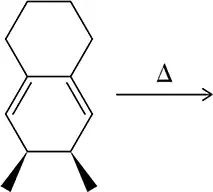
For the following electrocyclic reactions, did the substituents move in a conrotatory or disrotatory direction? Would you use heat or light to cause movement in this direction?
(a)
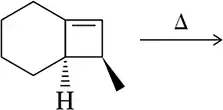
Problem 38c
Given the conditions, would you expect conrotatory or disrotatory ring closing/opening? Justify this on the basis of the molecular orbital picture.
(c)
Problem 39c
Draw the products you’d expect to form in Assessment 22.38.
(c)
Problem 40a
Predict the product of the Cope reactions shown.
(a)
Problem 40c
Predict the product of the Cope reactions shown.
(c)
Problem 41b
Predict the product of the Claisen reactions shown.
(b)
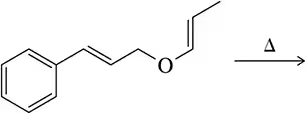
Problem 41c
Predict the product of the Claisen reactions shown.
(c)
Problem 43a
Predict the product of the following reactions. [When all of the reactions from this chapter are shown together, you must first decide which type of reaction each is. Is it a Diels–Alder, an electrocyclic, or a sigmatropic rearrangement? Drawing the product will be easier once this determination is made.]
(a)
Problem 43b
Predict the product of the following reactions. [When all of the reactions from this chapter are shown together, you must first decide which type of reaction each is. Is it a Diels–Alder, an electrocyclic, or a sigmatropic rearrangement? Drawing the product will be easier once this determination is made.]
(b)
Problem 44c
Suggest a diene and a dienophile that would give the following products.
(c)
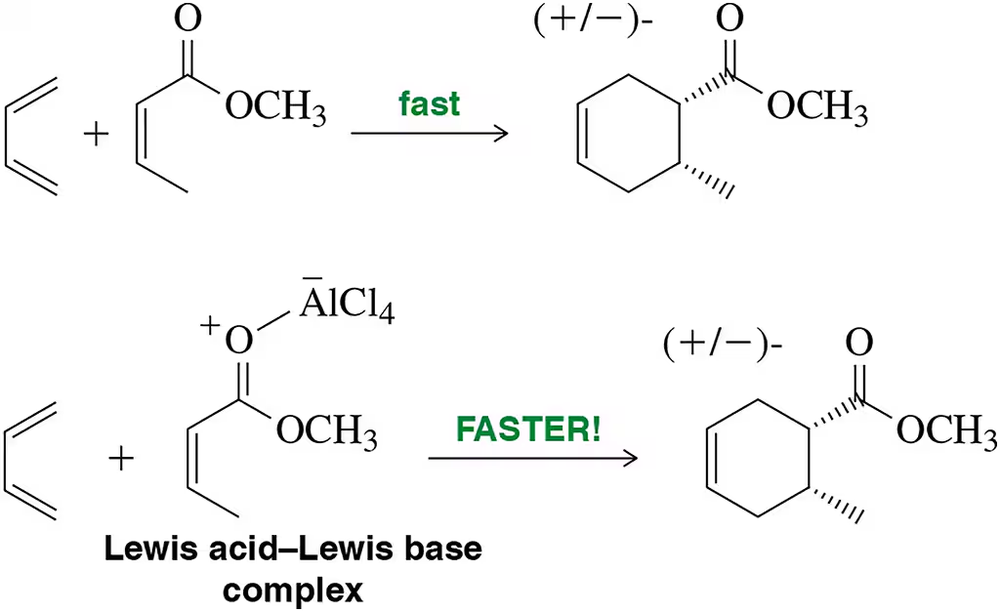
Problem 46
By forming a Lewis acid–Lewis base complex with the dienophile, Lewis acids are able to increase the rate of Diels–Alder reactions. Why might this be true?
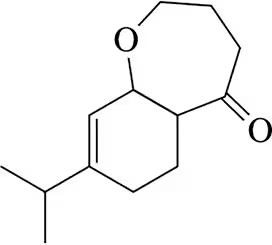
Problem 50
The [2 + 2] cyclization is especially useful when done intramolecularly.
(a) What makes intramolecular reactions more favorable?
(b) Predict the product of the following cyclization (slight modification of a reaction from Angew. Chem. 2011, 50, 5149)
Problem 52
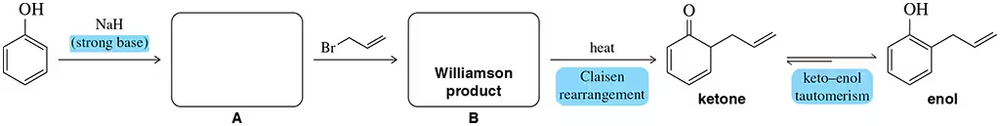
The Claisen rearrangement can be used to attach allyl groups to benzene via initial alkylation of a phenol.
(a) Predict the identity of A and B
(b) provide an arrow-pushing mechanism for each step. [The phenol alkylation is simply a Williamson ether synthesis reaction.]
Problem 53
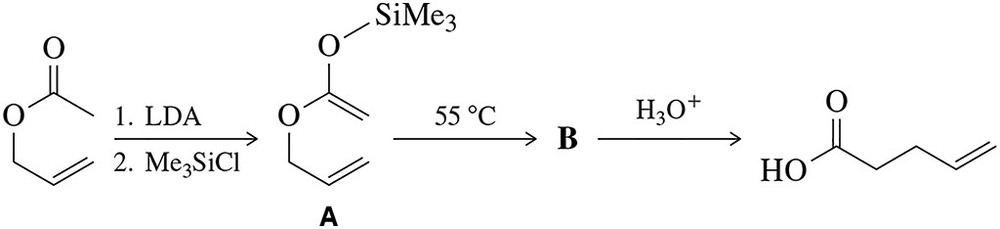
The Ireland–Claisen reaction is a variant of the Claisen reaction studied in this chapter.
(a) Suggest a mechanism for the first step.
(b) Predict the product (B) that would result from heating intermediate A.
(c) Hydrolysis of B gives a carboxylic acid.
Suggest a mechanism for this reaction.
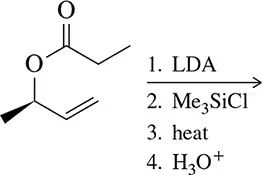
Problem 54a
Only after working Assessment 22.53, predict the product of the following reactions.
(a)
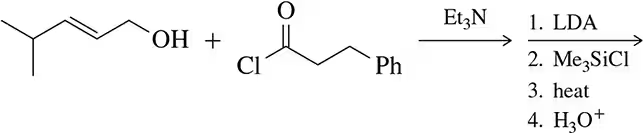
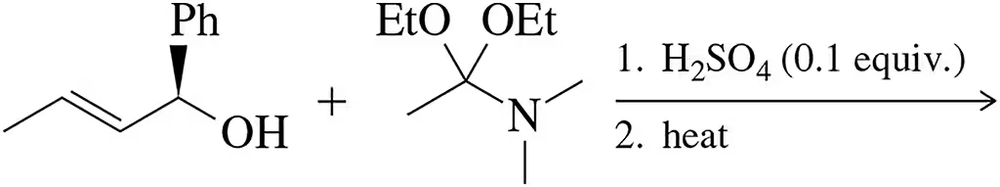
Problem 54b
Only after working Assessment 22.53, predict the product of the following reactions.
(b)
Problem 55
The Johnson–Claisen reaction is a variant of the Claisen reaction studied in this chapter. (a) Suggest a mechanism for the first step. (b) Predict the product (B) that would result from heating intermediate A.
Problem 56
The Eschenmoser–Claisen reaction is a variant of the Claisen reaction studied in this chapter. Based on your answers to Assessments 22.53–22.55, predict a product of this reaction.
Problem 58
In Chapter 20, we studied the aldol reaction. Although not discussed at the time, this reaction is stereospecific, proceeding through the Zimmerman–Traxler transition state shown here.
(a) Show an arrow-pushing mechanism for this concerted reaction.
(b) Why is this a favorable mechanism?
Problem 60
While not covered explicitly in this chapter, the ene reaction occurs similarly to the Diels–Alder reaction but replaces the electrons from one bond in the diene with the electrons in a C―H bond. Draw the mechanism for the following reaction. [Number the carbons and draw in the hydrogens of the product. And, of course, make a note of bonds formed and bonds broken.]
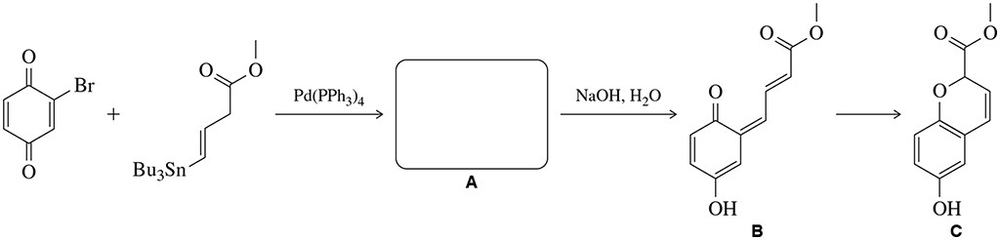
Problem 62
The product of the Stille coupling reaction (A) tautomerizes in a basic solution to give compound B. B spontaneously converts to C. (a) Propose a structure for A. (b) Suggest a mechanism for the conversion of A to B.
Problem 62c
The product of the Stille coupling reaction (A) tautomerizes in a basic solution to give compound B. B spontaneously converts to C.
(c) Suggest a mechanism for the conversion of B to C







![Chemical structure illustrating a [2 + 2] cyclization reaction with hydroxyl and methoxy groups, indicating photochemical conditions.](https://static.studychannel.pearsonprd.tech/courses/organic-chemistry/thumbnails/223f2cb8-866f-4e15-b954-21178f20d122)