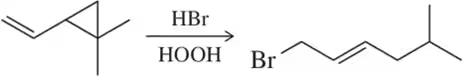Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 11 - Properties and Synthesis of Alkyl Halides: Radical Reactions
Ch. 11 - Properties and Synthesis of Alkyl Halides: Radical ReactionsProblem 52a
Provide the mechanism of the radical reactions shown.
(a)
Problem 52b
Provide the mechanism of the radical reactions shown.
(b)
Problem 53
One danger associated with storing ether solvents is their tendency to form explosive peroxides when exposed to oxygen. Suggest a mechanism by which the hydroperoxide might form. You can assume the presence of X• to start the reaction.
Problem 54
The following ethers are ranked according to their ability to form explosive peroxides. Explain this ranking based on your knowledge of the reaction mechanism.
Problem 55
The solvent tetrahydrofuran (THF) is often sold with a small amount of BHT added. Provide a mechanism that explains why this might be so.
Problem 56
If a small amount of a moderately nonpolar poisonous compound was added to a pond, why would it be safer to drink the water than it would be to eat the fish that live there?
Problem 57
Other molecules can be used as initiators in radical reactions. One such molecule is 2, 2'-azobisisobutyronitrile (AIBN). Show an arrow-pushing mechanism that rationalizes the formation of the following radical species. What are the driving forces of this reaction?
Problem 58
Tributyltin hydride (Bu3SnH) is often used as a 'radical carrier' in radical reactions. Which bond would you expect to be weaker, Sn–H or C–H? How might this relate to radical stability? Explain your answer.
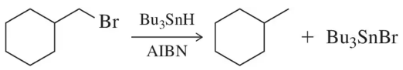
Problem 60
Haloalkanes can be reduced to alkanes using radical reactions involving Bu₃SnH and a catalytic amount of AIBN. Suggest a mechanism for this reaction. [Pay close attention to the bonds formed and broken in developing your mechanism.]
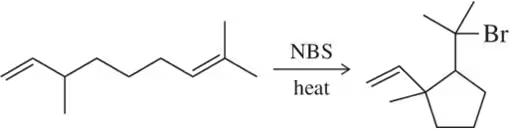
Problem 61
Cyclizations can be carried out under radical conditions involving Bu₃SnH and a catalytic amount of AIBN. Suggest a mechanism for this reaction. [Pay close attention to the bonds formed and broken in developing your mechanism. It may be helpful to number the carbons.]
Problem 62a
A halogenation intended to make compound A formed B instead.
(a) Suggest a mechanism for the intended formation of A.
Problem 62b
A halogenation intended to make compound A formed B instead.
(b) Suggest a mechanism that rationalizes the formation of B.
Problem 62c
A halogenation intended to make compound A formed B instead.
(c) Given the two mechanisms you drew, why might B have formed selectively?
Problem 62d
A halogenation intended to make compound A formed B instead.
(d) Without looking it up, would you expect C–Ha or C–Hb to have the lower bond-dissociation energy?
Problem 63a
Predict the product of the following benzylic bromination reactions.
(a)
Problem 63b
Predict the product of the following benzylic bromination reactions.
(b)
Problem 63c
Predict the product of the following benzylic bromination reactions.
(c)
Problem 64
The human body can excrete drugs and other exogenous molecules by converting them into polar, water-soluble compounds by a reaction similar to the autoxidation described in Section 11.6. Why are the following drug candidate molecules susceptible to oxidation by this pathway?