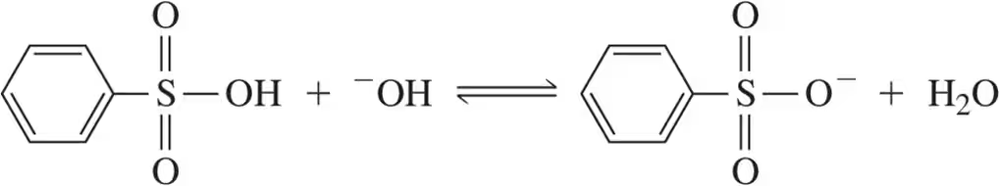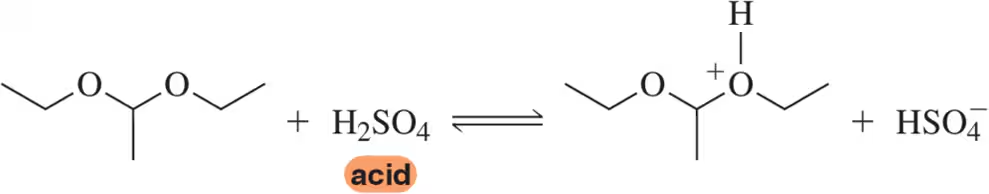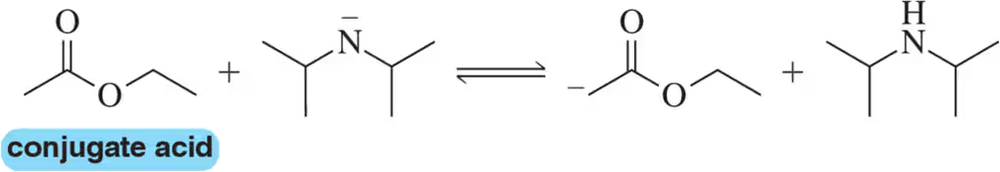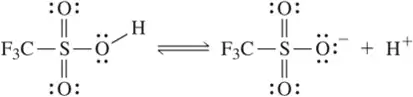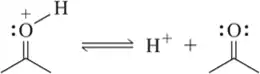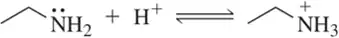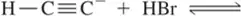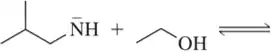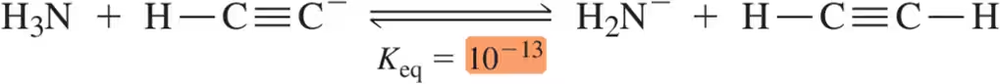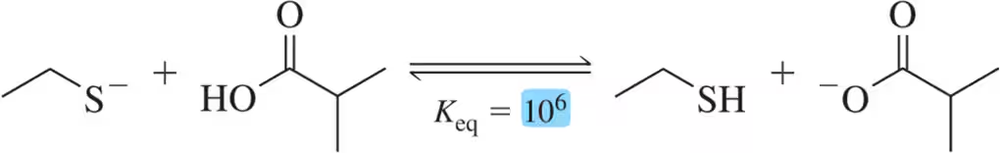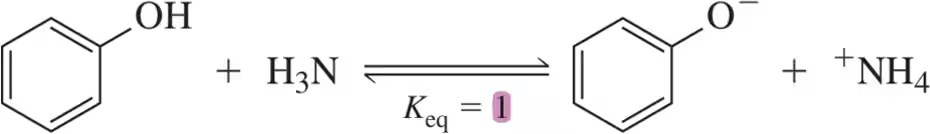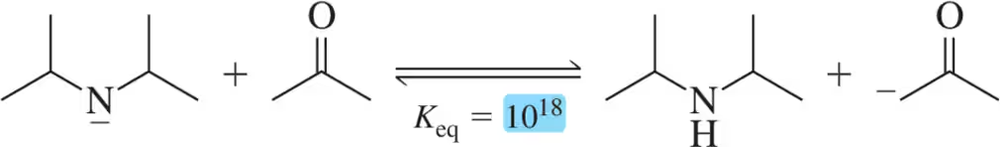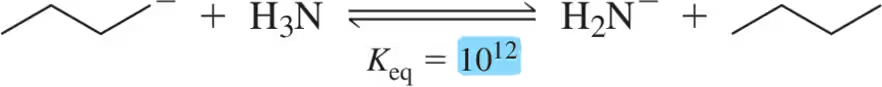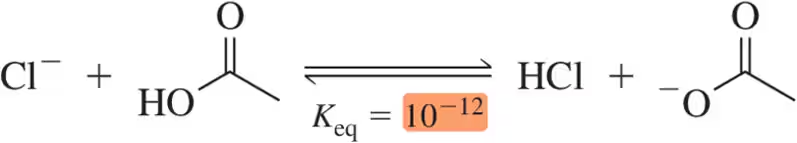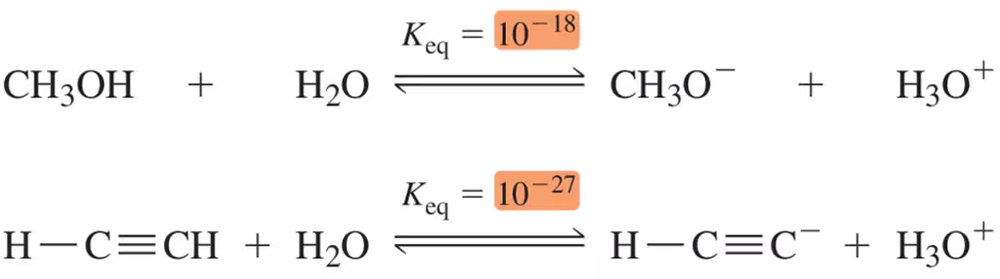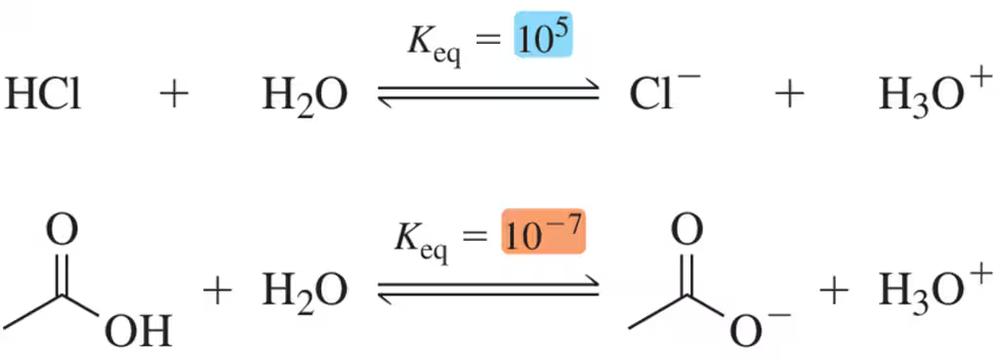Back
BackProblem 14c
Using the convention that the acid and base are on the left side of the chemical equation, label the acid, base, conjugate acid, and conjugate base in the following reactions.
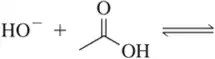
(c)
Problem 15a
Based on the one species that is identified for you, label the remaining molecules as acid, base, conjugate acid, or conjugate base.
(a)
Problem 15b
Based on the one species that is identified for you, label the remaining molecules as acid, base, conjugate acid, or conjugate base.
(b)
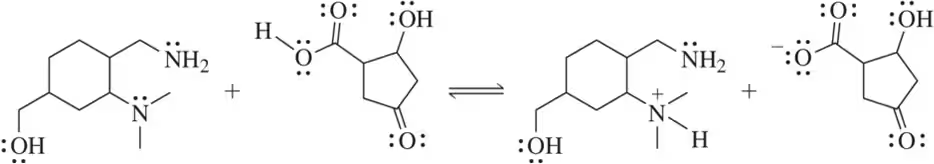
Problem 15c
Based on the one species that is identified for you, label the remaining molecules as acid, base, conjugate acid, or conjugate base.
(c)
Problem 16a
Provide an arrow-pushing mechanism for the following hypothetical acid half-reactions. [These are only intended to help you learn about arrow pushing in acid–base reactions.]
(a)
Problem 16b
Provide an arrow-pushing mechanism for the following hypothetical acid half-reactions. [These are only intended to help you learn about arrow pushing in acid–base reactions.]
(b)
Problem 17b
Provide an arrow-pushing mechanism for the following hypothetical base half-reactions.
(b)
Problem 17c
Provide an arrow-pushing mechanism for the following hypothetical base half-reactions.
(c)
Problem 18c
Provide an arrow-pushing mechanism for the following acid–base reactions.
(c)
Problem 18d
Provide an arrow-pushing mechanism for the following acid–base reactions.
(d)
Problem 19
Acid–base reactions are reversible. Show a mechanism for the reverse of the reactions in Assessment 4.18.
Problem 20a(iv,v)
In the following reactions,
(iv) provide an arrow-pushing mechanism of the proton transfer that will occur, and
(v) predict the product of the reactions. [You'll need to provide the lone pairs here.]
(a)
Problem 20a(i,ii,iii)
In the following reactions,
(i) identify the acid and base,
(ii) identify the most electron-rich atom in the base,
(iii) identify the most acidic hydrogen in the acid,
(a)
Problem 20b(i,ii,iii)
In the following reactions,
(i) identify the acid and base,
(ii) identify the most electron-rich atom in the base,
(iii) identify the most acidic hydrogen in the acid,
(b)
Problem 20b(iv,v)
In the following reactions,
(iv) provide an arrow-pushing mechanism of the proton transfer that will occur, and
(v) predict the product of the reactions. [You'll need to provide the lone pairs here.]
(b)
Problem 20c(i,ii,iii)
In the following reactions,
(i) identify the acid and base,
(ii) identify the most electron-rich atom in the base,
(iii) identify the most acidic hydrogen in the acid,
(c)
Problem 20c(iv,v)
In the following reactions,
(iv) provide an arrow-pushing mechanism of the proton transfer that will occur, and
(v) predict the product of the reactions. [You'll need to provide the lone pairs here.]
(c)
Problem 20d
In the following reactions,
(i) identify the acid and base,
(ii) identify the most electron-rich atom in the base,
(iii) identify the most acidic hydrogen in the acid,
(iv) provide an arrow-pushing mechanism of the proton transfer that will occur, and
(v) predict the product of the reactions. [You'll need to provide the lone pairs here.]
(d) H2O + HCl ⇌
Problem 21c
Write the Keq expression for the following acid–base reactions. [You don't need to calculate Keq here.]
(c)
Problem 22a
Given the value of Keq for the following acid–base reactions, identify the weakest acid and the weakest base.
(a)
Problem 22b
Given the value of Keq for the following acid–base reactions, identify the weakest acid and the weakest base.
(b)
Problem 22c
Given the value of Keq for the following acid–base reactions, identify the weakest acid and the weakest base.
(c)
Problem 23a
Given the Keq values for the following acid–base reactions, identify the strongest acid and the strongest base.
(a)
Problem 23b
Given the Keq values for the following acid–base reactions, identify the strongest acid and the strongest base.
(b)
Problem 23c
Given the Keq values for the following acid–base reactions, identify the strongest acid and the strongest base.
(c)
Problem 24
An unknown base (B⁻) has been identified as very weak. What does this tell you about the strength of its conjugate acid, HB? Is it stable or unstable? Is it reactive or unreactive?
Problem 25a
An unknown acid (HA) has been identified as very strong. What does this tell you about the stability of the conjugate base, A⁻? Is it strong or weak? Is it reactive or unreactive?
Problem 26
Design an acid–base extraction scheme to separate a mixture of the basic amine N,N-dimethylaniline and naphthalene.
Problem 28
Given the following Keq values, how much stronger of an acid is methanol (CH3OH) than acetylene (HC≡CH)?
Problem 29
Given the following Keq values, how much stronger of a base is acetate (CH3CO2-) than chloride (Cl⁻)?