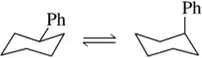Back
BackProblem 35
All things being equal, would you expect a first-order reaction to be faster or slower than a second-order reaction?
Problem 36
Third-order reactions are rare. Why do you think that is?
Problem 37a
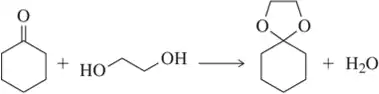
Calculate ∆H° for the following reactions.
(a)
Problem 37b
Calculate ∆H° for the following reactions.
(b) CH3Br + HCl → CH3Cl + HBr
Problem 37c
Calculate ∆H° for the following reactions.
(c) CH3CH3 + HOOH → CH3CH2OH + H2O
Problem 38a
Using bond-dissociation energies, identify the most stable radical. Justify the difference in stability based on the structure.
(a)
Problem 38c
Using bond-dissociation energies, identify the most stable radical. Justify the difference in stability based on the structure.
(c)
Problem 38d
Using bond-dissociation energies, identify the most stable radical. Justify the difference in stability based on the structure.
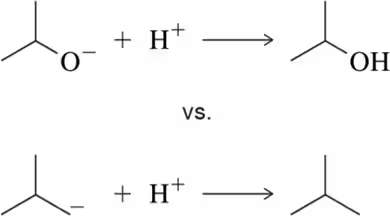
(d) I• vs •OH
Problem 39
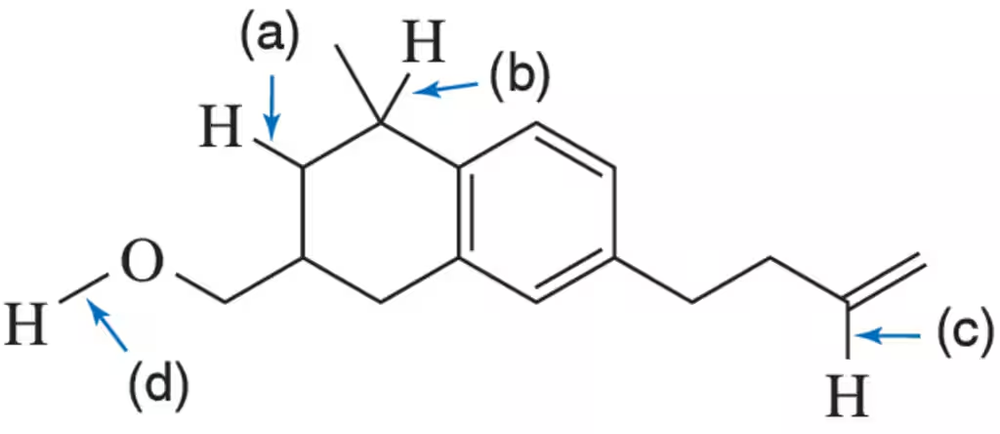
Give the approximate bond-dissociation energy for each indicated bond.
Problem 40a
For each pair of reactions, predict which will happen more quickly.
[For (a) and (b), think about the stability of the bases involved.]
(a)
Problem 40c
For each pair of reactions, predict which will happen more quickly. [For (c) and (d), the more substituted carbocation is more stable due to hyperconjugation.]
(c)
Problem 41(a)
The following are all substitution reactions, two of which we study in later chapters. With no knowledge of mechanism, what would you expect the ratio of products to be for each reaction, based on a random statistical distribution?
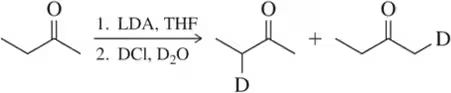
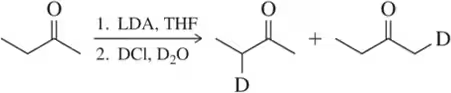
(a) Replacing a hydrogen (H) with deuterium (D):
Problem 41a
The following are all substitution reactions, two of which we study in later chapters. With no knowledge of mechanism, what would you expect the ratio of products to be for each reaction, based on a random statistical distribution?
(a) Replacing a hydrogen (H) with chlorine (Cl):
Problem 41b
The following are all substitution reactions, two of which we study in later chapters. With no knowledge of mechanism, what would you expect the ratio of products to be for each reaction, based on a random statistical distribution?
(b) Replacing a hydrogen (H) with bromine (Br):
Problem 42
Given the ratio of products obtained in the bromination of propane, calculate the relative reactivity of a 1° C–H bond to a 2° C–H bond under these conditions.
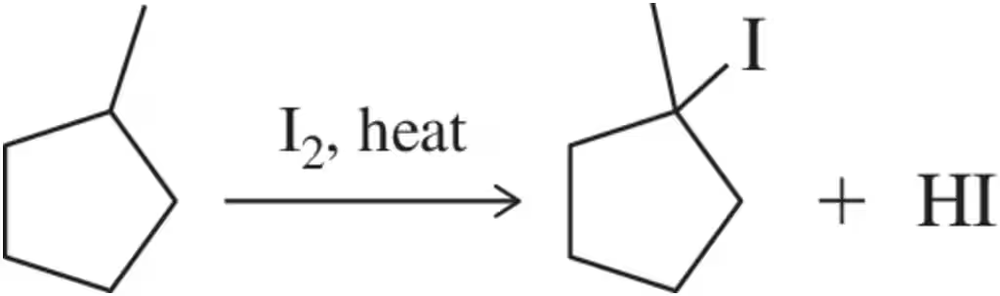
Problem 43b
Through the course of this chapter, we have discussed only alkane chlorination and bromination, yet there are two other halogens we have not discussed.
(b) Is radical iodination a favorable reaction? Do you expect it to be selective? Show your calculations.
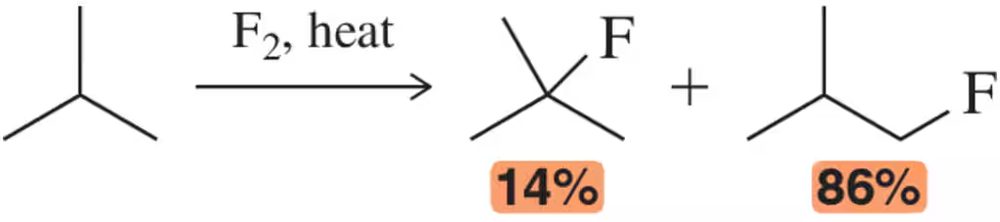
Problem 45a
The radical fluorination of 2-methyl propane resulted in a 14:86 ratio of products.
(a) On the basis of this ratio, calculate the relative reactivity of 1° and 3° C―H bonds in the radical fluorination.
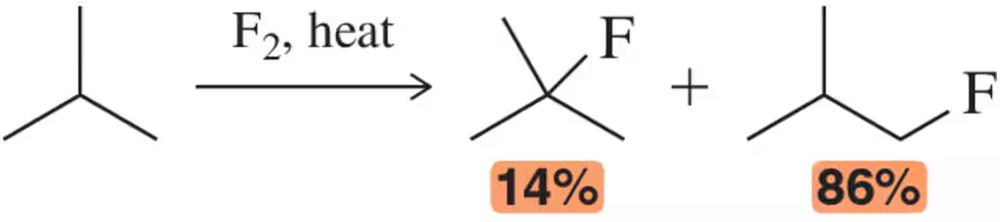
Problem 45b
The radical fluorination of 2-methyl propane resulted in a 14:86 ratio of products.
(b) From the relative reactivity, calculate the difference in energy between the transition states of the first propagation steps leading to a 1° and 3° radical.
Problem 46a
Predict the major monohalogenation product(s) of the following reactions. Indicate whether you think the reaction will be selective and justify your position.
(a)
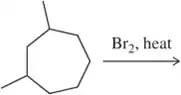
Problem 46c
Predict the major monohalogenation product(s) of the following reactions. Indicate whether you think the reaction will be selective and justify your position.
(c)
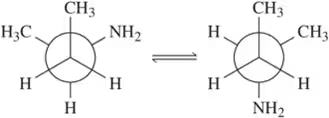
Problem 47c
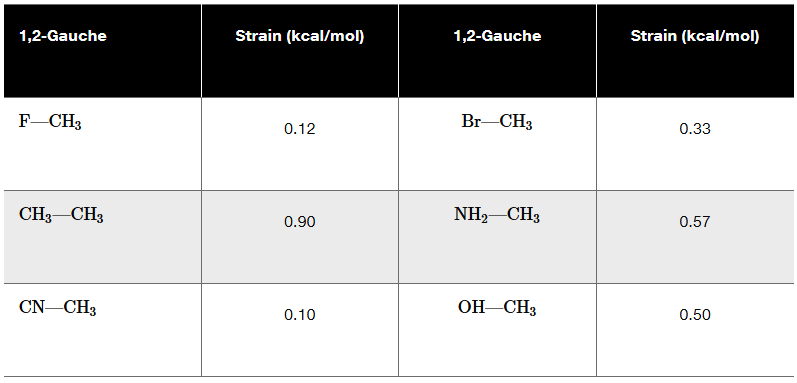
The following table of strain energies is associated with a variety of 1,2-gauche interactions. Use this table to answer the questions (i)–(iv).
For each of the bond rotations shown, (i) identify which you believe to be more stable, (ii) calculate ∆G°, (iii) calculate the equilibrium constant, and (iv) draw the transition state.
(c)
Problem 48a(i)
For each of the following acid–base reactions, (i) predict which side of the reaction you expect to be favored. If a pKa is not one of the ten common ones we learned in Chapter 4, it will be given to you.
(a)
Problem 48a(ii)
For each of the following acid–base reactions, (ii) calculate Keq. If a pKa is not one of the ten common ones we learned in Chapter 4, it will be given to you.
(a)
Problem 48a(iii)
For each of the following acid–base reactions, (iii) calculate ∆G°. If a pKa is not one of the ten common ones we learned in Chapter 4, it will be given to you.
(a)
Problem 48a(iv)
For each of the following acid–base reactions, (iv) draw the transition state, paying close attention to the degree of bond forming and breaking present in the transition state. If a pKa is not one of the ten common ones we learned in Chapter 4, it will be given to you.
(a)
Problem 49a
For each of the following reactions, identify the bonds that are broken and formed. Be sure to indicate whether the bond that is broken is a σ bond or a π bond.
(a)
Problem 49c
For each of the following reactions, identify the bonds that are broken and formed. Be sure to indicate whether the bond that is broken is a σ bond or a π bond.
(c)
Problem 49e
For each of the following reactions, identify the bonds that are broken and formed. Be sure to indicate whether the bond that is broken is a σ bond or a π bond.
(e)
Problem 50a
The A value of a substituent on a cyclohexane ring is essentially the ∆G° for a substituent going from the equatorial to the axial position in a chair–chair interconversion. Because most substituents prefer to be in the equatorial position, A values are, by definition, positive numbers. Use the table of A values to calculate ∆G° and Keq for the chair–chair interconversions shown.
(a)
Problem 50b
The A value of a substituent on a cyclohexane ring is essentially the ∆G° for a substituent going from the equatorial to the axial position in a chair–chair interconversion. Because most substituents prefer to be in the equatorial position, A values are, by definition, positive numbers. Use the table of A values to calculate ∆G° and Keq for the chair–chair interconversions shown.
(b)